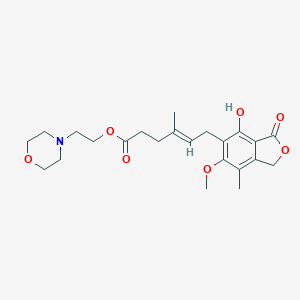
-
吗替麦考酚酯
- names:
Mycophenolate Mofetil
- CAS号:
128794-94-5
MDL Number: MFCD00867568 - MF(分子式): C23H31NO7 MW(分子量): 433.49
- EINECS:680-628-5 Reaxys Number:
- Pubchem ID:5281078 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM001238-100mg | 100mg | 99.7% | ¥ 1002.00 | ¥ 1002.00 | 2-3天 | ¥ 0.00 | ||
| YZM001238-50mg | 50mg | 99.7% | ¥ 585.00 | ¥ 585.00 | 2-3天 | ¥ 0.00 |
| 中文别名 | 吗替麦考酚酯(128794-94-5,Mycophenolate Mofetil);麦考酚酸酯;霉酚酸酯;霉酚酸吗啉乙酯 |
| 英文别名 | Mycophenolate Mofetil(128794-94-5);Mycophenolatemofetil;TM-MMF |
| CAS号 | 128794-94-5 |
| Inchi | InChI=1S/C23H31NO7/c1-15(5-7-19(25)30-13-10-24-8-11-29-12-9-24)4-6-17-21(26)20-18(14-31-23(20)27)16(2)22(17)28-3/h4,26H,5-14H2,1-3H3/b15-4+ |
| InchiKey | RTGDFNSFWBGLEC-SYZQJQIISA-N |
| 分子式 Formula | C23H31NO7 |
| 分子量 Molecular Weight | 433.49 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度≥ 100 mg/mL(230.69 mM)*"≥" means soluble可溶, but saturation unknown溶解度未知. |
| 性状 | 白色至灰白色固体粉末 |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / 溶液中:-80°C 6 months月 -20°C 1 month月 |
吗替麦考酚酯(128794-94-5,Mycophenolate Mofetil)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染
Mycophenolate Mofetil(128794-94-5) Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:吗替麦考酚酯(128794-94-5,Mycophenolate Mofetil),吗替麦考酚酯试剂,吗替麦考酚酯的纯度,吗替麦考酚酯的质量,吗替麦考酚酯的外观,吗替麦考酚酯的作用,吗替麦考酚酯的厂家,吗替麦考酚酯的合成路线,吗替麦考酚酯的MSDS,吗替麦考酚酯的溶解度,吗替麦考酚酯的生产
| 产品说明 | 吗替麦考酚酯(128794-94-5,Mycophenolate Mofetil)是麦考酚酸(MPA)的吗啉代乙酯,具有有效的免疫抑制特性。 |
| Introduction | Mycophenolate Mofetil(128794-94-5,吗替麦考酚酯) is the morpholinoethyl ester of mycophenolic acid (MPA) with potent immunosuppressive properties. |
| Application1 | 吗替麦考酚酯通过选择性抑制嘌呤生物合成的从头途径来停止T细胞和B细胞增殖。 |
| Application2 | Mycophenolate Mofetil is a non-competitive, selective and reversible inhibitor of inosine monophosphate dehydrogenase I/II with IC50 of 39 nM and 27 nM, respectively. |
| Application3 | Mycophenolate mofetil is a carboxylic ester resulting from the formal condensation between the carboxylic acid group of mycophenolic acid and the hydroxy group of 2-(morpholin-4-yl)ethanol. |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| 象形图 |    |
|---|---|
| 信号警告 | Danger |
| GHS危险说明 |
Aggregated GHS information provided by 52 companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (98.08%): Harmful if swallowed [Warning Acute toxicity, oral] H341 (11.54%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H360 (19.23%): May damage fertility or the unborn child [Danger Reproductive toxicity] H400 (90.38%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (13.46%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
| 防范说明代码 |
P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P391, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Effizienz von Mycophenolat-Mofetil bei der Therapie der intermedi?ren und posterioren Uveitis(Der Ophthalmologe,2002) |
| Stellungnahme zum Einsatz von Mycophenolat-Mofetil beim systemischen Lupus erythematodes(Zeitschrift für Rheumatologie,2013) |
| Effect of RS61443 in combination with leflunomide or FK506 on rat heart allograft survival(Transplant International,1996) |
| RS-61443 reverses acute renal allograft rejection in dogs(Transplant International Official Journal of the European Society for Organ Transplantation,1992) |
| Mycophenolat scheint Azathioprin in der Erhaltungstherapie bei Lupusnephritis überlegen(Zeitschrift für Rheumatologie,2012) |
1.Nocturnal Arousal in a 68-Year-Old Woman.
Hoque R, DelRosso LM. J Clin Sleep Med. 2016 Mar 21. pii: jc-00024-16. [Epub ahead of print]
ABSTRACT: The patient is a 68-year-old white woman, with past medical history of autoimmune hepatitis, hypertension, coronary artery disease, depression, and mild cognitive impairment. Medications include: mycophenolate mofetil, lisinopril, aspirin, sertraline, trazadone, and galantamine. She presents for evaluation of witnessed snoring and sleep maintenance insomnia, with frequent tossing and turning during the night. She goes to bed at 9:30 PM and falls asleep within minutes. She has two nocturnal awakenings, usually to urinate. She falls asleep quickly upon returning from the bathroom. She rises at 6 AM, and feels refreshed upon awakening. She denies lower extremity restlessness, or leg kicking during sleep. She has been told she snores, but denies witnessed apnea. She denies use of tobacco, alcohol, or illicit drugs.
2.Pharmacokinetic Variability of Mycophenolic Acid in Pediatric and Adult Patients with Hematopoietic Stem Cell Transplantation.
Zhang D1, Renbarger JL2, Chow DS1. J Clin Pharmacol. 2016 Apr 6. doi: 10.1002/jcph.745. [Epub ahead of print]
The aim of this study was to evaluate the pharmacokinetic variations of mycophenolic acid (MPA), the active metabolite of mycophenolate mofetil (MMF), in both pediatric and adult patients following hematopoietic stem cell transplantation (HSCT). Twenty pediatric patients with a median age of 3 years (range, 0.2-12 years) and thirteen adult patients with a median age of 54 years (range, 18-63 years) were enrolled. Blood samples were collected on days 0, 7, 14, 21 and 30 after allogeneic HSCT. Total and free (unbound) MPA, as well as MPAG were quantified using a validated LC-MS/MS assay. The plasma protein binding of MPA and MPAG did not change significantly in pediatric patients over the one month sampling period post HSCT. However, it increased in adult patients from day 7 to day 30 post HSCT, from 97.3±0.8% to 98.3±0.6% for MPA (P <0.05), and 74.6±9.4% to 82.9±8.1% for MPAG (P <0.05). The plasma protein binding of MPA was significantly higher in males compared to females in both pediatric (98.
3.Everolimus versus mycophenolate mofetil de novo after lung transplantation - a prospective, randomized, open-label trial.
Strueber M1, Warnecke G2,3, Fuge J4, Simon AR5, Zhang R2, Welte T3,4, Haverich A2,3, Gottlieb J3,4. Am J Transplant. 2016 Apr 22. doi: 10.1111/ajt.13835. [Epub ahead of print]
The role of mTOR inhibitors in de novo immunosuppression after lung transplantation is not well-defined. We compared Everolimus versus mycophenolate mofetil in an investigator-initiated single center trial in Hannover, Germany. A total of 190 patients were randomly assigned 1:1 on day 28 post-transplantation to MMF or Everolimus combined with CsA and Steroids. Patients were followed up for two years. Primary endpoint was freedom from BOS. Secondary endpoints were incidence of acute rejections, infections, treatment failure and kidney function. BOS-free survival in ITT analysis was similar in both groups (p=0.174). The study protocol was completed by 51% of enrolled patients. The per-protocol analysis shows incidence of BOS- 1/43 in the everolimus and 8/54 in the MMF group (p=0.041). Less biopsy-proven AR (p=0.005), CMV antigenemia (p=0.005), lower respiratory tract infection (p=0.003) and no leucopenia were seen in the everolimus group. GFR decreased in both groups about 50% within 6 months.
4.Enhancement of Mycophenolate Mofetil Permeation for Topical Use by Eucalyptol and N-Methyl-2-pyrrolidone.
Amnuaikit T1, Songkram C2, Pinsuwan S1. Scientifica (Cairo). 2016;2016:9672718. doi: 10.1155/2016/9672718. Epub 2016 Mar 16.
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid (MPA) which can be metabolized by esterase. MMF has been approved by the United States Food and Drug Administration (USFDA) for treatment of psoriasis patient with skin symptoms. However, it remains unclear whether MMF is efficiently effective to treat skin symptoms developed from psoriasis. The insufficient amount of MMF penetrating through the skin results in the treatment failure due to the difficulty in MMF penetration through the stratum corneum. Skin permeation enhancers such as eucalyptol (EUL) and N-methyl-2-pyrrolidone (NMP) potentially aid in increasing skin penetration. This study aimed to investigate the effects of a concentration ratio (% w/v) between two enhancers (EUL and NMP). The results showed that EUL enhanced MMF permeation with an enhancement ratio (ER) of 3.44 while NMP was not able to promote the penetration of MMF. Interestingly, the synergistic effect of the two enhancers was observed with a suitable ratio given that the ER was 8.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



