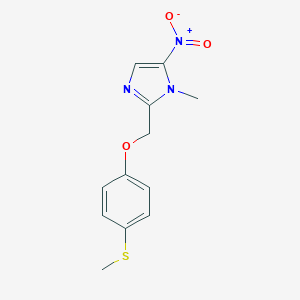
-
非昔硝唑
- names:
Fexinidazole
- CAS号:
59729-37-2
MDL Number: - MF(分子式): C12H13N3O3S MW(分子量): 279.31
- EINECS: Reaxys Number:
- Pubchem ID: Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000772-10mg | 10mg | 99.92% | ¥ 1518.00 | ¥ 1518.00 | 2-3天 | ¥ 0.00 | ||
| YZM000772-5mg | 5mg | 99.92% | ¥ 926.25 | ¥ 926.25 | 2-3天 | ¥ 0.00 |
| 中文别名 | 非昔硝唑(Fexinidazole,59729-37-2),非辛达唑 |
| 英文别名 | Fexinidazole(非昔硝,59729-37-2) |
| CAS号 | 59729-37-2 |
| Inchi | InChI=1S/C12H13N3O3S/c1-14-11(13-7-12(14)15(16)17)8-18-9-3-5-10(19-2)6-4-9/h3-7H,8H2,1-2H3 |
| InchiKey | MIWWSGDADVMLTG-UHFFFAOYSA-N |
| 分子式 Formula | C12H13N3O3S |
| 分子量 Molecular Weight | 279.31 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度50 mg/mL(179.01 mM;Need ultrasonic)H2O< 0.1 mg/mL(insoluble) |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:非昔硝唑MSDS,非昔硝唑蒸汽压,非昔硝唑合成,非昔硝唑标准,非昔硝唑应用,非昔硝唑合成,非昔硝唑沸点,非昔硝唑闪点,非昔硝唑用途,非昔硝唑溶解度,非昔硝唑价格,非昔硝唑作用,非昔硝唑结构式,非昔硝唑用处,非昔硝唑毒理性质,非昔硝唑物理性质
| 产品说明 | 非昔硝唑(Fexinidazole,59729-37-2) 一种5-硝基咪唑药物 |
| Introduction | Fexinidazole(非昔硝,59729-37-2)Fexinidazole is a 5-nitroimidazole drug currently in clinical |
| Application1 | Fexinidazole is a 5-nitroimidazole drug currently in clinical development for the treatment of human sleeping sickness (human African trypanosomiasis [HAT]), caused by infection with species of the pr |
| Application2 | Fexidazole can produce active amines with indirect toxicity and mutagenic effects on trypanosomes. It is active in vitro against Trypanosoma gambiae and many other trypanosoma subspecies (including Tr |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Fexinidazole: First Global Approval Drugs 2019/PMID: 30635838 |
| Determination of an Optimal Dosing Regimen for Fexinidazole, a Novel Oral Drug for the Treatment of Human African Trypanosomiasis: First-in-Human Studies Clinical Pharmacokinetics 2014/PMID: 24535888 |
| Heterogeneity in the in vitro susceptibility of Loa loa microfilariae to drugs commonly used in parasitological infections Parasites & Vectors 2018/PMID: 29615094 |
| Systemic and Target-Site Pharmacokinetics of Antiparasitic Agents Clinical Pharmacokinetics 2020/PMID: 32100246 |
| Evaluation of the in vitro trypanocidal activity of methylated flavonoid constituents of Vitex simplicifolia leaves BMC Complementary and Alternative Medicine 2015/PMID: 25886869 |
Toosendanin relatives, trypanocidal principles from Meliae Cortex
Abstract
Africa Trypanosomiasis remains a serious health problem, but the approved drugs for this disease are so few that novel trypanocidal compounds are demanded. In search for trypanocidal principles from medicinal plants, we found MeOH extracts of Meliae Cortex with potent activity through the screening from about 300 kinds of methanolic extract. By bioassay-guided fractionation from this extract through the liquid-liquid partition and subsequent chromatographic technique using silica gel and ODS, finally we disclosed toosendanin (1) and its relatives as active principles. These active congeners showed not only potent trypanocidal activity but also little cytotoxicity to display the excellent selective index. Taking the isolated amount as well as trypanocidal activity into consideration, 1 was disclosed to be the responsible active principle in Meliae Cortex. Additionally, the derivatives of 1 were chemically prepared from 1 and bioactivity of them were also evaluated. Through the comparison with their trypanocidal activity among the isolated relatives and the synthesized derivatives of 1, the epoxide moiety was revealed to be essential for their potent trypanocidal activity. Furthermore, 3-O-acetyl group and 7-hydroxyl group were presumed to be important functional groups and introduction of methylpropionyl group into hemiacetal hydroxy moiety was clarified to enhance their typanocidal activity.
Fexinidazole: First Global Approval
Abstract
Fexinidazole Winthrop (hereafter referred to as fexinidazole) is a DNA synthesis inhibitor developed by the Drugs for Neglected Diseases initiative (DNDi), in collaboration with Sanofi, for the oral treatment of human African trypanosomiasis (HAT) [commonly known as 'sleeping sickness'] and Chagas' disease. The drug is a 5-nitroimidazole derivative first discovered by Hoechst AG (now part of Sanofi) and was identified by the DNDi in 2005 as having activity against Trypanosoma brucei gambiense and T. b. rhodesiense. Under Article 58 of Regulation (EC) no. 726/2004 (a regulatory mechanism for reviewing new medicines destined for use outside of the EU), fexinidazole has been granted a positive opinion by the EMA for the treatment of both the first-stage (haemo-lymphatic) and second-stage (meningo-encephalitic) of HAT due to T. b. gambiense (g-HAT) in adults and children aged ≥ 6 years and weighing ≥ 20 kg. This approval will facilitate and support marketing authorization application in endemic countries in 2019; following registration, fexinidazole will be distributed via the WHO to endemic countries for g-HAT. Phase 3 evaluation of fexinidazole for g-HAT is ongoing in the Democratic Republic of the Congo and Guinea and the drug is also in development for Chagas' disease, with a study currently ongoing in Spain. Clinical development for visceral leishmaniasis is discontinued. This article summarizes the milestones in the development of fexinidazole leading to this first approval for g-HAT.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



