-
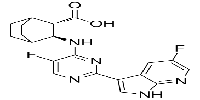
匹莫迪韦
- names:
Pimodivir
- CAS号:
1629869-44-8
MDL Number: MFCD31382121 - MF(分子式): C20H19F2N5O2 MW(分子量): 399.39
- EINECS: Reaxys Number:
- Pubchem ID:67286591 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000729-5mg | 5mg | 99.04% | ¥ 1569.00 | ¥ 1569.00 | 2-3天 | ¥ 0.00 | ||
| YZM000729-2mg | 2mg | 99.04% | ¥ 965.25 | ¥ 965.25 | 2-3天 | ¥ 0.00 |
| 中文别名 | 匹莫迪韦(1629869-44-8);吡莫地韦;VX-787;VX 787;VX787; JNJ-872;JNJ 872;JNJ872;VRT-0928787; VRT 0928787;VRT0928787;吡莫地尔; (2S,3S)-3-((5-氟-2-(5-氟-1H-吡咯并[2,3-b]吡啶-3-基)嘧啶-4-基)氨基)双环[2.2.2 ]辛烷-2-羧酸; |
| 英文别名 | Pimodivir(1629869-44-8);VX-787;VX-787; VX 787; VX787; JNJ-872; JNJ 872; JNJ872; VRT-0928787; VRT 0928787; VRT0928787;pimodivir; (2S,3S)-3-((5-Fluoro-2-(5-fluoro-1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)bicyclo[2.2.2]octane-2-carboxylic Acid; |
| CAS号 | 1629869-44-8 |
| Inchi | InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 |
| InchiKey | JGPXDNKSIXAZEQ-SBBZOCNPSA-N |
| 分子式 Formula | C20H19F2N5O2 |
| 分子量 Molecular Weight | 399.39 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度100 mg/mL(250.38 mM;Need ultrasonic) |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | 存放在阴凉干燥处,短期(数天至数周)在0-4°C下,长期(数月至数年)在-20°C下保存。 |
匹莫迪韦(1629869-44-8,Pimodivir,VX-787)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:匹莫迪韦试剂,匹莫迪韦杂质,匹莫迪韦中间体,匹莫迪韦密度,匹莫迪韦纯度,匹莫迪韦闪点,匹莫迪韦熔点,匹莫迪韦合成,匹莫迪韦溶解度,匹莫迪韦购买,
| 产品说明 | 匹莫迪韦(1629869-44-8,Pimodivir,VX-787)是一种有效的可口服的甲型流感病毒聚合酶的抑制剂,通过抑制PB2亚基起作用 |
| Introduction | 匹莫迪韦(1629869-44-8,Pimodivir,VX-787)is an orally bioavailable inhibitor of influenza A virus polymerasesthrough interaction with the viralPB2subunit. |
| Application1 | Pimodivir(VX-787)是一种可口服的甲型流感病毒聚合酶抑制剂,通过抑制PB2亚基作用。 |
| Application2 | 已发现吡咯并吡啶衍生物VX-787是一种抗病毒药,目前仍处于针对甲型流感病毒感染的II期试验中。 |
| Application3 |
Pimodivir是A型流感病毒聚合酶复合物的聚合酶基础蛋白2(PB2)亚基的口服生物可利用的非核苷抑制剂,具有潜在的抗病毒活性。给药后,匹莫地韦占据PB2 的7-甲基GTP(m7GTP)结合位点,从而阻断流感聚合酶复合物的夺帽活性并抑制病毒mRNA的合成。PB2是组成甲型流感病毒聚合酶复合物的三个亚基之一,它负责感染细胞核中病毒RNA(vRNA)基因组的复制和转录。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Smee DF, et al. Activities of JNJ63623872 and GS 4071 against influenza A H1N1pdm and H3N2 virus infections in mice. Antiviral Res. 2016 Dec;136:45-50. |
| Fu Y, et al. JNJ872 inhibits influenza A virus replication without altering cellular antiviral responses. Antiviral Res. 2016 Sep;133:23-31. |
| Boyd MJ, et al. Isosteric replacements of the carboxylic acid of drug candidate VX-787: Effect of charge on antiviral potency and kinase activity of azaindole-based influenza PB2 inhibitors. Bioorg Me |
| Byrn RA, et al. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Chemother. 2015 Mar;59(3):1569-82. |
| A Phase 2 Study of Pimodivir (JNJ-63623872) in Combination with Oseltamivir in Elderly and NonElderly Adults Hospitalized with Influenza A Infection: OPAL study PMID 32604406; The Journal of infectiou |
匹莫迪韦(1629869-44-8,Pimodivir,VX-787)参考文献:
1.Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit.
Byrn RA;Jones SM;Bennett HB;Bral C;Clark MP;Jacobs MD;Kwong AD;Ledeboer MW;Leeman JR;McNeil CF;Murcko MA;Nezami A;Perola E;Rijnbrand R;Saxena K;Tsai AW;Zhou Y;Charifson PS Antimicrob Agents Chemother. 2015 Mar;59(3):1569-82. doi: 10.1128/AAC.04623-14. Epub 2014 Dec 29.
VX-787 is a novel inhibitor of influenza virus replication that blocks the PB2 cap-snatching activity of the influenza viral polymerase complex. Viral genetics and X-ray crystallography studies provide support for the idea that VX-787 occupies the 7-methyl GTP (m(7)GTP) cap-binding site of PB2. VX-787 binds the cap-binding domain of the PB2 subunit with a KD (dissociation constant) of 24 nM as determined by isothermal titration calorimetry (ITC). The cell-based EC50 (the concentration of compound that ensures 50% cell viability of an uninfected control) for VX-787 is 1.6 nM in a cytopathic effect (CPE) assay, with a similar EC50 in a viral RNA replication assay. VX-787 is active against a diverse panel of influenza A virus strains, including H1N1pdm09 and H5N1 strains, as well as strains with reduced susceptibility to neuraminidase inhibitors (NAIs). VX-787 was highly efficacious in both prophylaxis and treatment models of mouse influenza and was superior to the neuraminidase inhibitor, oseltamivir, including in delayed-start-to-treat experiments, with 100% survival at up to 96 h postinfection and partial survival in groups where the initiation of therapy was delayed up to 120 h postinfection.
2.Pimodivir treatment in adult volunteers experimentally inoculated with live influenza virus: a Phase IIa, randomized, double-blind, placebo-controlled study.
Trevejo JM;Asmal M;Vingerhoets J;Polo R;Robertson S;Jiang Y;Kieffer TL;Leopold L Antivir Ther. 2017 Dec 15. doi: 10.3851/IMP3212. [Epub ahead of print]
BACKGROUND: ;Pimodivir (formerly JNJ-63623872) is a novel, non-nucleoside polymerase complex inhibitor with in vitro activity against influenza A virus, including pandemic 2009 H1N1, H7N9, H5N1 strains as well as neuraminidase- and amantadine-resistant strains.;METHODS: ;Randomized, double-blind, placebo-controlled, Phase IIa study. Healthy volunteers (n=104) were inoculated with an influenza A/Wisconsin/67/2005 (H3N2) challenge virus. 72 received pimodivir and 32 placebo. Pimodivir was dosed for 5 days once daily from 24 h after viral inoculation at four dose levels: 100 mg, 400 mg, loading dose 900/600 mg and loading dose 1,200/600 mg.;RESULTS: ;Pimodivir significantly reduced viral shedding (area under the concentration versus time curve [AUC] measured by 50% tissue culture infective dose [TCID;50;] or qRT-PCR) versus placebo as measured by cell culture assay in the pooled analysis (Jonckheere-Terpstra dose-response trend test [P=0.036]). Reductions were observed in viral shedding (AUC, duration and peak measured by grade), influenza-like symptoms (AUC, duration and peak measured by grade) and clinical symptoms (duration and peak measured by grade) for all pimodivir groups versus placebo, significantly so for the 1,200/600 mg group.
3.JNJ872 inhibits influenza A virus replication without altering cellular antiviral responses.
Fu Y;Gaelings L;Söderholm S;Belanov S;Nandania J;Nyman TA;Matikainen S;Anders S;Velagapudi V;Kainov DE Antiviral Res. 2016 Sep;133:23-31. doi: 10.1016/j.antiviral.2016.07.008. Epub 2016 Jul 20.
JNJ-63623872 (formally known as VX-787; referred to here as JNJ872) is an orally bioavailable compound, which is in phase II clinical trials for the treatment of influenza A virus (IAV) infections. Here we show that JNJ872 inhibits at nanomolar concentrations the transcription of viral RNA in IAV-infected human macrophages by targeting a highly conserved site on the cap-snatching domain of influenza polymerase basic 2 protein (PB2). Furthermore, even lower concentrations of JNJ872 protected macrophages from IAV-mediated death when given in combination with 100 nM gemcitabine, which also attenuated transcription and replication of viral RNA. Importantly, treating human macrophages with JNJ872 allowed expression of many immune-related and other genes, involved in antiviral responses, such as indoleamine 2,3-dioxygenase 1 (IDO), and cytosolic 5'-nucleotidase 3A (NT5C3A). Moreover, our targeted metabolomics analysis indicate that treatment with JNJ782 did not interfere with metabolic responses to infection, which further supported our transcriptomics results. Thus, VX-737 alone or in combination with other drugs could be beneficial for treating IAV infected patients, because it would allow the development of antiviral responses and, thereby, protect individuals from current and future infections with closely related IAV strains.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



