-
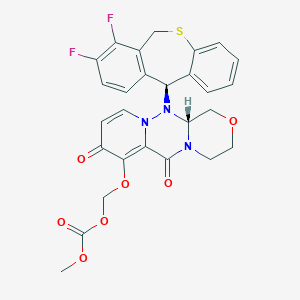
巴洛沙韦酯
- names:
Baloxavir marboxil
- CAS号:
1985606-14-1
MDL Number: MFCD31619272 - MF(分子式): C27H23F2N3O7S MW(分子量): 571.55
- EINECS: Reaxys Number:
- Pubchem ID:124081896 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000726-5mg | 5mg | 99.95% | ¥ 3240.00 | ¥ 3240.00 | 2-3天 | ¥ 0.00 | ||
| YZM000726-1mg | 1mg | 99.95% | ¥ 1170.00 | ¥ 1170.00 | 2-3天 | ¥ 0.00 |
| 中文别名 | 巴洛沙韦酯(1985606-14-1);巴洛沙韦马波地尔;玛巴洛沙韦; |
| 英文别名 | Baloxavir marboxil(1985606-14-1); S-033188;HY-109025;(((12aR)-12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12ahexahydro-1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazin-7-yl)oxy)methyl methyl carbonate;10.1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazine-6,8-dione, 12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-3,4,12,12a-tetrahydro-7-hydroxy-, (12aR)-;baloxavir;baloxavir marboxil;Xofluza; |
| CAS号 | 1985606-14-1 |
| Inchi | InChI = 1S / C27H23F2N3O7S / c1-36-27(35)39-14-38-25-19(33)8-9-31-24(25)26(34)30-10-11-37-12- 21(30)32(31)23-15-6-7-18(28)22(29)17(15)13-40-20-5-3-2-4-16(20)23 / h2- 9,21,23H,10-14H2,1H3 / t21-,23 + / m1 / s1 |
| InchiKey | RZVPBGBYGMDSBG-GGAORHGYSA-N |
| 分子式 Formula | C27H23F2N3O7S |
| 分子量 Molecular Weight | 571.55 |
| 溶解度Solubility | |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
巴洛沙韦酯(1985606-14-1,S-033188,Baloxavir)毒理性质:
In clinical trials, there was little evidence that baloxavir caused liver injury, either in the form of serum enzyme elevations or clinically apparent liver disease. A proportion of patients with acute influenza A may have minor serum enzyme elevations during the acute illness, but these are independent of therapy and do not appear to be exacerbated by baloxavir.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Ld50 (oral, rats): >2000 mg/kg [MSDS] **Pregnancy Risk** There are no available data on the use of this drug in pregnant women to predict or inform a drug-associated risk of adverse developmental outcomes. However, there are known risks to the mother and fetus associated with influenza virus infection during pregnancy. In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits with oral administration of Baloxavir marboxil at exposures approximately 5 (rats) and 7 (rabbits) times the systemic Baloxavir exposure at the maximum recommended human dose [FDA label]. The estimated background risk of major birth defects and miscarriage for the indicated population is not known at this time [FDA label]. **Breastfeeding** There are no data on the presence of this drug in human breastmilk, the effects on the breastfed infant, or the effects on milk production. Baloxavir and its related metabolites were present in the milk of lactating rats [FDA label]. **Carcinogenicity** Carcinogenicity studies have not been completed with baloxavir marboxil [FDA label]. **Mutagenesis** Baloxavir marboxil and the active metabolite, baloxavir, were not shown to be mutagenic in in-vitro and in in-vivo genotoxicity assays, which included bacterial mutation assays in S. typhimurium and E. coli, micronucleus tests with cultured mammalian cells, and in the rodent micronucleus assay [FDA label]. **Impairment of Fertility** In a fertility and early embryonic development study in rats, doses of baloxavir marboxil at 20, 200, or 1,000 mg/kg/day were given to female animals for 2 weeks before mating, during mating and until day 7 of pregnancy. Male animals were dosed for 4 weeks before mating and throughout mating. There were no measured effects on fertility, mating performance, or early embryonic development at any dose level, resulting in systemic drug exposure (AUC) approximately 5 times the MRHD (maximum recommended human dose) [FDA label].
巴洛沙韦酯(1985606-14-1,S-033188,Baloxavir)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:巴洛沙韦酯试剂,巴洛沙韦酯杂质,巴洛沙韦酯中间体,巴洛沙韦酯密度,巴洛沙韦酯合成,巴洛沙韦酯闪点,巴洛沙韦酯溶解度,巴洛沙韦酯旋光度,巴洛沙韦酯结构式,巴洛沙韦酯MSDS,
| 产品说明 | 巴洛沙韦酯(1985606-14-1,S-033188,Baloxavir)是流感帽依赖性核酸内切酶的选择性抑制剂,可阻止聚合酶功能 |
| Introduction | 巴洛沙韦酯(1985606-14-1,S-033188,Baloxavir) marboxil is a small molecule inhibitor of the capependent endonuclease of influenza A and B viruses. |
| Application1 | |
| Application2 | |
| Application3 |
巴洛沙韦酯(1985606-14-1,S-033188,Baloxavir)药理学:
1、Baloxavir marboxil是流感帽依赖性核酸内切酶的选择性抑制剂,可阻止聚合酶功能,从而阻止流感病毒mRNA复制[L1475],[A39907]。它已显示出对甲型和乙型流感病毒感染的治疗活性,包括对当前抗病毒药具耐药性的菌株[A39894]。该药物抑制病毒复制所需的酶,从而迅速治疗流感病毒感染[L1475],[FDA标签],并减轻与感染有关的症状。已显示,单剂这种药物在缓解流感症状方面优于安慰剂,在病毒学结局(以病毒载量降低为标志)方面优于奥司他韦和安慰剂药物[A39894]。曲妥昔洛韦的安全性优于奥司他韦一剂[L1475],[A39894],使其成为治疗流感病毒的有效治疗选择。
2、巴洛沙韦酯是抗病毒剂,巴洛沙韦酯可用于预防或治疗病毒性疾病的药物。它们可能发挥作用的方式包括通过抑制病毒DNA聚合酶来防止病毒复制。与特定的细胞表面受体结合并抑制病毒渗透或脱壳;抑制病毒蛋白质合成;或阻止病毒组装的后期.
3、巴洛沙韦酯是酶抑制剂,巴洛沙韦酯与酶结合的化合物或试剂,可防止正常的底物-酶结合和催化反应。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Frederick G Hayden, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med. 2018 Sep 6;379(10):913-923. |
| Hideyuki Ikematsu, et al. Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts. N Engl J Med. 2020 Jul 23;383(4):309-320. |
| Cap-dependent Endonuclease Inhibitor S-033188 for the Treatment of Influenza: Results from a Phase 3, Randomized, Double-Blind, Placebo- and Active-Controlled Study in Otherwise Healthy Adolescents an |
| Heo YA: Baloxavir: First Global Approval. Drugs. 2018 Apr;78(6):693-697. doi: 10.1007/s40265-018-0899-1. [PMID:29623652] |
| Dong LH, Cao XR: Studies of the Interaction of Influenza Virus RNA Polymerase PAN with Endonuclease Inhibitors. Interdiscip Sci. 2018 Jun;10(2):430-437. doi: 10.1007/s12539-017-0239-2. Epub 2017 Jun 1 |
1、Cap-dependent Endonuclease Inhibitor S-033188 for the Treatment of Influenza: Results from a Phase 3, Randomized, Double-Blind, Placebo- and Active-Controlled Study in Otherwise Healthy Adolescents and Adults with Seasonal Influenza
Simon Portsmouth, MD,1 Keiko Kawaguchi, MS,2 Masatsugu Arai, MS,3 Kenji Tsuchiya, MS,2 and Takeki Uehara, PhD2
Abstract Background Cap-dependent endonuclease (CEN) resides in the PA subunit of influenza virus polymerase and mediates the “cap-snatching” process during viral mRNA biosynthesis. S-033188 is a potent, selective, small molecule inhibitor of CEN. Here we report clinical and virologic outcomes from a global Phase 3 study CAPSTONE-1. Method This was a multicenter, randomized, double-blind, placebo- and active-controlled study. Key eligibility criteria included 12–64 years of age, fever (axillary temperature ≥38.0°C), ≥1 general symptom and ≥1 respiratory symptom (moderate to severe), and ≤48 hours from symptom onset. Patients between 20 and 64 years of age were randomized in 2:2:1 ratio to receive a single oral administration of S-033188, placebo, or 75 mg oseltamivir BID for 5 days. Patients between 12 and 19 years of age were randomized in 2:1 ratio to receive either a single oral administration of S-033188 or placebo. The primary efficacy endpoint was time to alleviation of influenza symptoms (TTAS) in the infected intent to treat population. Viral titer and RNA content were determined from pre- and postdose nasal/throat swabs. Result A total of 1436 patients were randomized. TTAS was significantly shorter in the S-033188 group than that in the placebo group (median TTAS: 53.7 hours vs. 80.2 hours, P < 0.0001). Median time to cessation of viral shedding was 24 hours in patients treated with S-033188, compared with 72 hours in those treated with oseltamivir (P < 0.0001) and 96 hours for placebo (P < 0.0001). Patients in the S-033188 group had significantly greater reductions from baseline in both viral titer and RNA content than those in oseltamivir or placebo groups at all time-points until Day 3 (compared with oseltamivir) or Day 5 (compared with placebo). S-033188 was generally well tolerated, with overall incidence of treatment-emergent adverse events lower than that seen with oseltamivir.
2、Antivirals in medical biodefense
J. J. Bugert, F. Hucke, P. Zanetta, M. Bassetto & A. Brancale
Abstract The viruses historically implicated or currently considered as candidates for misuse in bioterrorist events are poxviruses, filoviruses, bunyaviruses, orthomyxoviruses, paramyxoviruses and a number of arboviruses causing encephalitis, including alpha- and flaviviruses. All these viruses are of concern for public health services when they occur in natural outbreaks or emerge in unvaccinated populations. Recent events and intelligence reports point to a growing risk of dangerous biological agents being used for nefarious purposes. Public health responses effective in natural outbreaks of infectious disease may not be sufficient to deal with the severe consequences of a deliberate release of such agents. One important aspect of countermeasures against viral biothreat agents are the antiviral treatment options available for use in post-exposure prophylaxis. These issues were adressed by the organizers of the 16th Medical Biodefense Conference, held in Munich in 2018, in a special session on the development of drugs to treat infections with viruses currently perceived as a threat to societies or associated with a potential for misuse as biothreat agents. This review will outline the state-of-the-art methods in antivirals research discussed and provide an overview of antiviral compounds in the pipeline that are already approved for use or still under development.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



