-
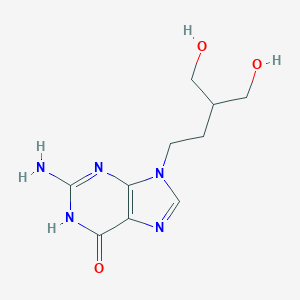
喷昔洛韦
- names:
Penciclovir
- CAS号:
39809-25-1
MDL Number: MFCD00866931 - MF(分子式): C10H15N5O3 MW(分子量): 253.26
- EINECS:663-371-3 Reaxys Number:
- Pubchem ID:135398748 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000713-100mg | 100mg | >98.0% | ¥ 1113.00 | ¥ 1113.00 | 2-3天 | ¥ 0.00 | ||
| YZM000713-50mg | 50mg | >98.0% | ¥ 696.15 | ¥ 696.15 | 2-3天 | ¥ 0.00 |
| 中文别名 | 喷昔洛韦(39809-25-1);9-(4-羟基-3-羟甲基丁-1-基)鸟嘌呤;西雷亚尔39123;RL-39123;那佛;昔洛韦;昔洛韦,钠盐;昔洛韦,一钠盐,一水合物;克塔韦尔; |
| 英文别名 | Penciclovir(39809-25-1); BRL-39123; BRL 39123; BRL39123; VSA 671; VSA671; VSA-671; Penciclovir; Denavir, Vectavir and Fenivir;9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine;RL 39123;RL-39123;enavir;enciclovir;enciclovir Visfarm;enciclovir, monosodium salt;enciclovir, monosodium salt, monohydrate;ectavir; |
| CAS号 | 39809-25-1 |
| Inchi | InChI = 1S / C10H15N5O3 / c11-10-13-8-7(9(18)14-10)12-5-15(8)2-1-6(3-16)4-17 / h5-6, 16-17H,1-4H2,(H3,11,13,14,18) |
| InchiKey | JNTOCHDNEULJHD-UHFFFAOYSA-N |
| 分子式 Formula | C10H15N5O3 |
| 分子量 Molecular Weight | 253.26 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度25 mg/mL(98.71 mM;Need ultrasonic) |
| 性状 | 白色结晶固体粉末,Power |
| 储藏条件 Storage conditions | -20°C Freezer, 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)毒理性质:
Symptoms of overdose include headache, abdominal pain, increased serum lipase, nausea, dyspepsia, dizziness, and hyperbilirubinemia.
Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention.
Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination。
Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam . Use proparacaine hydrochloride to assist eye irrigation .
喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:喷昔洛韦试剂,喷昔洛韦杂质,喷昔洛韦合成,喷昔洛韦中间体,喷昔洛韦溶解度,喷昔洛韦密度,喷昔洛韦旋光度,喷昔洛韦闪点,喷昔洛韦购买,喷昔洛韦结构式,
| 产品说明 | 喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)是抗病毒剂用于治疗多种疱疹病毒感染.具有毒性低, |
| Introduction | 喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)is reported to be potent againstHSVtypes 1 and 2 withIC50of 0.04.8 μg/mL and 0.06.4 μg/mL, respectively. |
| Application1 | Penciclovir有效作用于HSV 1和2,IC50 分别为0.04-1.8μg/ mL和0.06-4.4μg/ mL。 |
| Application2 | 喷昔洛韦是一种鸟苷类似物抗病毒药物,用于治疗各种疱疹病毒感染。它是一种核苷类似物,具有低毒性和良好的选择性。 |
| Application3 |
喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)药理学:
※喷昔洛韦是一种具有抗病毒活性的合成无环鸟嘌呤衍生物,主要用于治疗1型和2型单纯疱疹病毒(HSV)的感染。在被HSV感染的细胞中,喷昔洛韦被病毒胸苷激酶磷酸化,随后被细胞激酶转化为活性代谢产物,喷昔洛韦三磷酸,通过阻断脱氧鸟苷三磷酸底物的结合,竞争性抑制病毒HSV聚合酶。结果,选择性抑制了疱疹病毒DNA的合成和复制。
※喷昔洛韦,也称为地那韦或PCV,属于被称为次黄嘌呤的有机化合物。次黄嘌呤是含有嘌呤衍生物1H-purin-6(9H)-1的化合物。嘌呤是一个的由二环芳香族化合物嘧啶稠合至环的咪唑环。喷昔洛韦是一种药物,用于治疗因各种疱疹病毒感染而在嘴唇和面部反复发作的唇疱疹。喷昔洛韦以固体存在,微溶(在水中)和弱酸性化合物(基于其pKa)。已在多种生物流体(如尿液和血液)中检测到喷昔洛韦。在细胞内,喷昔洛韦主要位于细胞质中。喷昔洛韦可以由鸟嘌呤生物合成。
※喷昔洛韦是鸟嘌呤的2-氨基嘌呤类成员,其中第9位的氢被4-羟基-3-(羟甲基)丁-1-基取代。一种抗病毒药,局部给药以治疗唇疱疹。前药泛昔洛韦用于口服给药。它具有抗病毒药的作用。它是2-氨基嘌呤的成员和丙烷-1,3-二醇的成员。它来自鸟嘌呤。
※喷昔洛韦(Penciclovir)是鸟嘌呤类似物类抗病毒药物, 用于治疗多种疱疹病毒感染。具有毒性低,病毒敏感性高等特点。喷昔洛韦口服吸收低,常用于局部给药。 泛昔洛韦是喷昔洛韦的前药,口服吸收好。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)危害标识:
| 象形图 | |
| 信号 | Warning |
| GHS危险说明 | H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] |
| Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. | |
| 防范说明代码 | P264, P280, P305+P351+P338, and P337+P313 |
| (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Piret J, et al. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011 Feb;55(2):459-72. |
| Xiong Z, et al. Imaging chemically modified adenovirus for targeting tumors expressing integrin alphavbeta3 in living micewith mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J |
| Antiviral Consideration for Transplantation Including Drug Resistance Principles and Practice of Transplant Infectious Diseases 2019 |
| Phenotypic and Genotypic Testing of HSV-1 and HSV-2 Resistance to Antivirals Herpes Simplex Virus 2020 31617182 |
| Pharmacokinetics and Pharmacodynamics of Antiviral Drugs in Special Population Principles and Practice of Transplant Infectious Diseases 2019 |
喷昔洛韦(39809-25-1,Penciclovir,BRL 39123,VSA 671)参考文献:
1.Clinical and antiviral effect of a single oral dose of famciclovir administered to cats at intake to a shelter.
Litster AL1, Lohr BR2, Bukowy RA2, Thomasy SM3, Maggs DJ3. Vet J. 2015 Feb;203(2):199-204. doi: 10.1016/j.tvjl.2014.11.011. Epub 2014 Nov 25.
Although famciclovir is efficacious in feline herpesvirus type 1 (FHV-1)-infected cats, effects of a single dose early in disease course have not been reported. In this two part, randomized, masked, placebo controlled study, cats received a single dose of 125 mg famciclovir (n = 43) or placebo (n = 43; pilot study), or 500 mg famciclovir (n = 41) or placebo (n = 40; clinical trial) on entering a shelter. FHV-1 PCR testing was performed, bodyweight and food intake were recorded, and signs of respiratory disease were scored prior to and 7 days following treatment. FHV-1 DNA was detected in 40% of cats in both parts at study entry. In the pilot study, ocular and nasal discharge scores increased from days 1 to 7 in famciclovir and placebo treated cats. Sneezing scores increased and bodyweight decreased in famciclovir-treated cats. The proportion of cats in which FHV-1 DNA was detected increased over time in all cats in the pilot study. In the clinical trial, food intake and median clinical disease scores for nasal discharge and sneezing increased from days 1 to 7 in both groups and demeanor scores worsened in famciclovir-treated cats.
2.Ribavirin inhibits human parainfluenza virus type 2 replication in vitro.
Kihira S1, Uematsu J, Kawano M, Itoh A, Ookohchi A, Satoh S, Maeda Y, Sakai K, Yamamoto H, Tsurudome M, O'Brien M, Komada H. Microbiol Immunol. 2014 Nov;58(11):628-35. doi: 10.1111/1348-0421.12192.
The antiviral activities of eight nucleoside analog antiviral drugs (ribavirin, acyclovir, lamivudine, 3'-azido-3'-deoxythymidine, emtricitabine, tenofovir, penciclovir and ganciclovir) against human parainfluenza virus type 2 (hPIV-2) were investigated. Only ribavirin (RBV) inhibited both cell fusion and hemadsorption induced by hPIV-2. RBV considerably reduced the number of viruses released from the cells. Virus genome synthesis was inhibited by RBV, as determined by real time PCR. An indirect immunofluorescence study showed that RBV largely inhibited viral protein synthesis. mRNAs of the proteins were not detected, indicating that inhibition of protein synthesis was caused by transcription inhibition by RBV. Using a recombinant green fluorescence protein-expressing hPIV-2 without matrix protein, it was found that RBV did not completely inhibit virus entry into the cells; however, it almost completely blocked multinucleated giant cell formation.
3.Cutaneous biodistribution of ionizable, biolabile aciclovir prodrugs after short duration topical iontophoresis: Targeted intraepidermal drug delivery.
Chen Y1, Zahui T1, Alberti I1, Kalia YN2. Eur J Pharm Biopharm. 2016 Feb;99:94-102. doi: 10.1016/j.ejpb.2015.11.010. Epub 2015 Nov 22.
The objective was to determine the cutaneous biodistribution of aciclovir (ACV), that is, the amount of drug as a function of depth within the skin following topical iontophoretic administration of amino acid ester prodrugs of ACV (ACV-X, where X=Arg, Ile or Val). The results were compared to those obtained with marketed formulations of aciclovir and penciclovir (PCV), and following topical iontophoresis of ACV. Quantification of molecules as a function of position in the skin was achieved by snap-freezing and cryotoming skin samples to obtain coarse or fine lamellae with a thickness of either 100μm or 20μm. The molecules - ACV, ACV-X or PCV - were extracted and quantified by validated UHPLC-MS/MS analytical methods. Passive delivery of ACV or PCV from marketed cream and ointment formulations after application for 60min resulted in modest cutaneous deposition (QDEP,ACV and QDEP,PCV<2nmol/cm(2)). Moreover, ACV and PCV were found mainly in the stratum corneum or superficial viable epidermis.
4.Indirect photochemical transformations of acyclovir and penciclovir in aquatic environments increase ecological risk.
An J1,2, Li G1, An T1, Nie X3. Environ Toxicol Chem. 2016 Mar;35(3):584-92. doi: 10.1002/etc.3238. Epub 2016 Feb 9.
Acyclovir and penciclovir, 2 antiviral drugs, are increasingly detected in aquatic environments. The present study explores the natural photochemical transformation mechanisms and fate of these drugs, examining direct and indirect photochemical transformation under simulated sunlight irradiation. The 2 antiviral drugs are photostable under certain conditions but significantly degrade in the presence of chromophoric dissolved organic matter (DOM). The degradation rate associated with the drugs' indirect photochemical transformation scaled with chromophoric DOM concentration. Quenchers and sensitizers were used to identify indirect photochemical transformation mechanism. Results suggested that both pharmaceuticals could be transformed by reacting with (1) O2 , (•) OH, and excited chromophoric DOM. The (1) O2 played an important role in indirect photochemical transformation. Furthermore, the reaction kinetics between their substructural molecules, guanine, isocytosine, and imidazole, with different reactive oxygen species were evaluated to determine which substrate functionalities were most susceptible to singlet oxygenation.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



