-
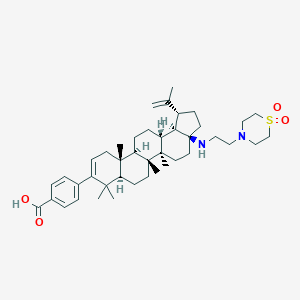
GSK3532795
- names:
GSK3532795
- CAS号:
1392312-45-6
MDL Number: - MF(分子式): C42H62N2O4S MW(分子量): 691.02
- EINECS: Reaxys Number:
- Pubchem ID:60152109 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000697-250mg | 250mg | ¥ 0.00 | ¥ 0.00 | Backorder | ¥ 0.00 | |||
| YZM000697-100mg | 100mg | >97% | ¥ 0.00 | ¥ 0.00 | 2-3天 | ¥ 0.00 |
| 中文别名 | GSK3532795(1392312-45-6);BMS-955176;BMS-955176 (GSK-3532795;GSK3532795;GSK3532795; |
| 英文别名 | GSK3532795(1392312-45-6);BMS-955176;BMS-955176 (GSK-3532795;GSK3532795;GSK3532795;BMS-955176(free base);benzoic acid, 4-(17-((2-(1,1-dioxido-4-thiomorpholinyl)ethyl)amino)-28-norlupa-2,20(29)-dien-3-yl)-;BMS-955176; |
| CAS号 | 1392312-45-6 |
| Inchi | No data available |
| InchiKey | No data available |
| 分子式 Formula | C42H62N2O4S |
| 分子量 Molecular Weight | 691.02 |
| 溶解度Solubility | |
| 性状 | Solid |
| 储藏条件 Storage conditions | 请根据产品建议的存储条件进行存储,Please store the product under the recommended condition sin the description. |
GSK3532795(1392312-45-6,BMS55176)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:GSK3532795试剂,GSK3532795杂质,GSK3532795中间体,GSK3532795密度,GSK3532795溶解度,GSK3532795旋光度,GSK3532795结构式,GSK3532795闪点,GSK3532795购买,
| 产品说明 | GSK3532795(1392312-45-6,BMS55176)是一种有效的、可口服、第二代HIV-1成熟的抑制剂。 |
| Introduction | GSK3532795(1392312-45-6,BMS55176) is a potent, orally active, secondenerationHIVmaturation inhibitor, withEC50s of 1.9, 10.2, 2.7 and 13 nM for HIV WT, HIV WT(human serum), |
| Application1 | HIV V370A, and HIV ΔV370, respectively. |
| Application2 | (1392312-45-6)GSK3532795 是一种有效的、可口服、第二代HIV-1成熟的抑制剂,对 HIV-1 WT,HIV-1 WT(人血清),HIV-1 V370A 和 HIV-1 ΔV370 的EC50值分别是 1.9,10.2,2.7 和 13 nM |
| Application3 |
GSK3532795(1392312-45-6,BMS55176)药理学:
(1392312-45-6)GSK3532795 是一种有效的、可口服、第二代HIV-1成熟的抑制剂,对 HIV-1 WT,HIV-1 WT(人血清),HIV-1 V370A 和 HIV-1 ΔV370 的EC50值分别是 1.9,10.2,2.7 和 13 nM
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| A Phase 2b Randomized, Active-Controlled, Double-Blind Trial to Investigate Safety, Efficacy and Dose-response of BMS-955176, Given on a Backbone of Tenofovir/Emtricitabine, in Treatment-Naive HIV-1 I |
| Randomized, Placebo-Controlled, Multiple-Dose Study to Evaluate the Pharmacodynamics, Safety and Pharmacokinetics of BMS-955176 (Double-Blinded) and BMS-955176 with Atazanavir +/- Ritonavir (Open-Labe |
| Study to Evaluate a HIV Drug for the Treatment of HIV Infection Phase 2 Completed 2019-11-25 |
| Dose-finding Study of BMS-955176 to Treat HIV-1 Infected Treatment-naive Adults Phase 2 Terminated 2018-09-19 |
| Strategy-confirming Study of BMS-955176 to Treat HIV-1 Infected Treatment-experienced Adults Phase 2 Terminated 2018-08-20 |
GSK3532795(1392312-45-6,BMS55176)参考文献:
1、Identification and Characterization of BMS-955176, a Second-Generation HIV-1 Maturation Inhibitor with Improved Potency, Antiviral Spectrum, and Gag Polymorphic Coverage
Beata Nowicka-Sans 1, Tricia Protack 1, Zeyu Lin 1, Zhufang Li 1, Sharon Zhang 1, Yongnian Sun 1, Himadri Samanta 1, Brian Terry 1, Zheng Liu 2, Yan Chen 2, Ny Sin 2, Sing-Yuen Sit 2, Jacob J Swidorski 2, Jie Chen 2, Brian L Venables 2, Matthew Healy 3, Nicholas A Meanwell 2, Mark Cockett 1, Umesh Hanumegowda 4, Alicia Regueiro-Ren 2, Mark Krystal 1, Ira B Dicker
Abstract BMS-955176 is a second-generation human immunodeficiency virus type 1 (HIV-1) maturation inhibitor (MI). A first-generation MI, bevirimat, showed clinical efficacy in early-phase studies, but ∼50% of subjects had viruses with reduced susceptibility associated with naturally occurring polymorphisms in Gag near the site of MI action. MI potency was optimized using a panel of engineered reporter viruses containing site-directed polymorphic changes in Gag that reduce susceptibility to bevirimat (including V362I, V370A/M/Δ, and T371A/Δ), leading incrementally to the identification of BMS-955176. BMS-955176 exhibits potent activity (50% effective concentration [EC50], 3.9 ± 3.4 nM [mean ± standard deviation]) toward a library (n = 87) of gag/pr recombinant viruses representing 96.5% of subtype B polymorphic Gag diversity near the CA/SP1 cleavage site. BMS-955176 exhibited a median EC50 of 21 nM toward a library of subtype B clinical isolates assayed in peripheral blood mononuclear cells (PBMCs). Potent activity was maintained against a panel of reverse transcriptase, protease, and integrase inhibitor-resistant viruses, with EC50s similar to those for the wild-type virus. A 5.4-fold reduction in EC50 occurred in the presence of 40% human serum plus 27 mg/ml of human serum albumin (HSA), which corresponded well to an in vitro measurement of 86% human serum binding. Time-of-addition and pseudotype reporter virus studies confirm a mechanism of action for the compound that occurs late in the virus replication cycle. BMS-955176 inhibits HIV-1 protease cleavage at the CA/SP1 junction within Gag in virus-like particles (VLPs) and in HIV-1-infected cells, and it binds reversibly and with high affinity to assembled Gag in purified HIV-1 VLPs. Finally, in vitro combination studies showed no antagonistic interactions with representative antiretrovirals (ARVs) of other mechanistic classes. In conclusion, BMS-955176 is a second-generation MI with potent in vitro anti-HIV-1 activity and a greatly improved preclinical profile compared to that of bevirimat.
2、Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity
Alicia Regueiro-Ren 1, Zheng Liu 1, Yan Chen 1, Ny Sin 1, Sing-Yuen Sit 1, Jacob J Swidorski 1, Jie Chen 1, Brian L Venables 1, Juliang Zhu 1, Beata Nowicka-Sans 1, Tricia Protack 1, Zeyu Lin 1, Brian Terry 1, Himadri Samanta 1, Sharon Zhang 1, Zhufang Li 1, Brett R Beno 1, Xiaohua S Huang 1, Sandhya Rahematpura 1, Dawn D Parker 1, Roy Haskell 1, Susan Jenkins 1, Kenneth S Santone 1, Mark I Cockett 1, Mark Krystal 1, Nicholas A Meanwell 1, Umesh Hanumegowda 1, Ira B Dicker
Abstract HIV-1 maturation inhibition (MI) has been clinically validated as an approach to the control of HIV-1 infection. However, identifying an MI with both broad polymorphic spectrum coverage and good oral exposure has been challenging. Herein, we describe the design, synthesis, and preclinical characterization of a potent, orally active, second generation HIV-1 MI, BMS-955176 (2), which is currently in Phase IIb clinical trials as part of a combination antiretroviral regimen.
3、Mechanistic Studies and Modeling Reveal the Origin of Differential Inhibition of Gag Polymorphic Viruses by HIV-1 Maturation Inhibitors
Zeyu Lin 1, Joseph Cantone 2, Hao Lu 2, Beata Nowicka-Sans 1, Tricia Protack 1, Tian Yuan 3, Hong Yang 3, Zheng Liu 4, Dieter Drexler 2, Alicia Regueiro-Ren 4, Nicholas A Meanwell 4, Mark Cockett 1, Mark Krystal 1, Max Lataillade 5, Ira B Dicker
Abstract HIV-1 maturation inhibitors (MIs) disrupt the final step in the HIV-1 protease-mediated cleavage of the Gag polyprotein between capsid p24 capsid (CA) and spacer peptide 1 (SP1), leading to the production of infectious virus. BMS-955176 is a second generation MI with improved antiviral activity toward polymorphic Gag variants compared to a first generation MI bevirimat (BVM). The underlying mechanistic reasons for the differences in polymorphic coverage were studied using antiviral assays, an LC/MS assay that quantitatively characterizes CA/SP1 cleavage kinetics of virus like particles (VLPs) and a radiolabel binding assay to determine VLP/MI affinities and dissociation kinetics. Antiviral assay data indicates that BVM does not achieve 100% inhibition of certain polymorphs, even at saturating concentrations. This results in the breakthrough of infectious virus (partial antagonism) regardless of BVM concentration. Reduced maximal percent inhibition (MPI) values for BVM correlated with elevated EC50 values, while rates of HIV-1 protease cleavage at CA/SP1 correlated inversely with the ability of BVM to inhibit HIV-1 Gag polymorphic viruses: genotypes with more rapid CA/SP1 cleavage kinetics were less sensitive to BVM. In vitro inhibition of wild type VLP CA/SP1 cleavage by BVM was not maintained at longer cleavage times. BMS-955176 exhibited greatly improved MPI against polymorphic Gag viruses, binds to Gag polymorphs with higher affinity/longer dissociation half-lives and exhibits greater time-independent inhibition of CA/SP1 cleavage compared to BVM. Virological (MPI) and biochemical (CA/SP1 cleavage rates, MI-specific Gag affinities) data were used to create an integrated semi-quantitative model that quantifies CA/SP1 cleavage rates as a function of both MI and Gag polymorph. The model outputs are in accord with in vitro antiviral observations and correlate with observed in vivo MI efficacies. Overall, these findings may be useful to further understand antiviral profiles and clinical responses of MIs at a basic level, potentially facilitating further improvements to MI potency and coverage.
4、Second Generation Inhibitors of HIV-1 Maturation
Alicia Regueiro-Ren 1, Ira B Dicker 2, Umesh Hanumegowda 2, Nicholas A Meanwell
Abstract The strategy and tactics subtending the discovery and development of the second generation HIV-1 maturation inhibitor GSK-3532795/BMS-955176, a compound that exhibits a broader spectrum of antiviral effect in vitro and in clinical studies than the prototypical maturation inhibitor bevirimat, are described.
5、Antiviral Activity, Safety, and Exposure-Response Relationships of GSK3532795, a Second-Generation Human Immunodeficiency Virus Type 1 Maturation Inhibitor, Administered as Monotherapy or in Combination With Atazanavir With or Without Ritonavir in a Phase 2a Randomized, Dose-Ranging, Controlled Trial (AI468002)
Carey Hwang 1, Dirk Schürmann 2 3, Christian Sobotha 2, Marta Boffito 4, Heather Sevinsky 1, Neelanjana Ray 1, Palanikumar Ravindran 1, Hong Xiao 1, Christian Keicher 2, Andreas Hüser 2, Mark Krystal 5, Ira B Dicker 5, Dennis Grasela 1, Max Lataillade
Abstract Background: GSK3532795 is a second-generation human immunodeficiency virus type 1 (HIV-1) maturation inhibitor that targets HIV-1 Gag, inhibiting the final protease cleavage between capsid protein p24 and spacer protein-1, producing immature, noninfectious virions. Methods: This was a phase 2a, randomized, dose-ranging multipart trial. In part A, subtype B-infected subjects received 5-120 mg GSK3532795 (or placebo) once daily for 10 days. In part B, subtype B-infected subjects received 40 mg or 80 mg GSK3532795 once daily with atazanavir (ATV) with or without (±) ritonavir (RTV) or standard of care (SOC) (tenofovir disoproxil fumarate 300 mg, emtricitabine 200 mg, and ATV/RTV 300 mg/100 mg) for 28 days. In part C, subtype C-infected subjects received 40 mg or 120 mg GSK3532795 once daily (or placebo) for 10 days. Endpoints included change in HIV-1 RNA from baseline on day 11 (parts A/C) or day 29 (part B). Results: A >1 log10 median decline in HIV-1 RNA was achieved by day 11 in parts A and C and day 29 in part B at GSK3532795 doses ≥40 mg; part B subjects receiving GSK3532795 and ATV ± RTV achieved similar declines to those receiving SOC. Median of the maximum declines in HIV-1 RNA were similar for the 40-120 mg once-daily dose groups regardless of baseline Gag polymorphisms. There were no deaths, adverse events leading to discontinuation, or serious adverse events. Conclusions: GSK3532795 demonstrated potent antiviral activity against subtype B (monotherapy or with ATV ± RTV) and subtype C, and was generally well tolerated, which supported continued development of GSK3532795 in subjects with HIV-1 subtype B or subtype C.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



