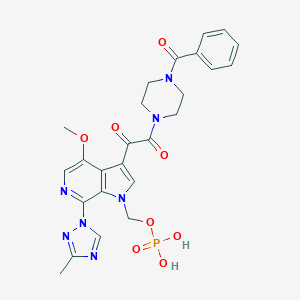
-
磷坦姆沙韦
- names:
Fostemsavir
- CAS号:
864953-29-7
MDL Number: MFCD23098796 - MF(分子式): C25H26N7O8P MW(分子量): 583.49
- EINECS:No data available Reaxys Number:No data available
- Pubchem ID:11319217 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000663-5mg | 5mg | 99.57% | ¥ 2156.00 | ¥ 2156.00 | 2-3天 | ¥ 0.00 | ||
| YZM000663-2mg | 2mg | 99.57% | ¥ 1170.00 | ¥ 1170.00 | 2-3天 | ¥ 0.00 |
| 中文别名 | 磷坦姆沙韦(864953-29-7),(3-((4-苄基-1-哌嗪基)(氧代)乙酰基)-4-甲氧基-7-(3-甲基-1H-1,2,4-三唑-1-基)-1H-吡咯并(2 ,3-c)吡啶-1-基)甲基磷酸二氢;1,2-乙二酮,1-(4-苯甲酰基-1-哌嗪基)-2-(4-甲氧基-7-(3-甲基-1H-1,2,4-三唑-1-基)-1-( (膦酰氧基)甲基)-1H-吡咯并(2,3-c)吡啶-3-基)-; |
| 英文别名 | Fostemsavir(864953-29-7),BMS-663068; BMS 663068;BMS663068;(3-((4-Benzoyl-1-piperazinyl)(oxo)acetyl)-4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo(2,3-c)pyridin-1-yl)methyl dihydrogen phosphate;1,2-Ethanedione, 1-(4-benzoyl-1-piperazinyl)-2-(4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1-((phosphonooxy)methyl)-1H-pyrrolo(2,3-c)pyridin-3-yl)-; |
| CAS号 | 864953-29-7 |
| Inchi | InChI = 1S / C25H26N7O8P / c1-16-27-14-32(28-16)23-21-20(19(39-2)12-26-23)18(13-31(21)15-40- 41(36,37)38)22(33)25(35)30-10-8-29(9-11-30)24(34)17-6-4-3-5-7-17 / h3- 7,12-14H,8-11,15H2,1-2H3,(H2,36,37,38) |
| InchiKey | SWMDAPWAQQTBOG-UHFFFAOYSA-N |
| 分子式 Formula | C25H26N7O8P |
| 分子量 Molecular Weight | 583.49 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度≥ 100 mg/mL(171.38 mM)H2O : 20 mg/mL(34.28 mM;Need ultrasonic)*"≥" means soluble可溶, but saturation unknown溶解度未知. |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)毒理性质:
磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:磷坦姆沙韦试剂,磷坦姆沙韦杂质,磷坦姆沙韦合成,磷坦姆沙韦中间体,磷坦姆沙韦密度,磷坦姆沙韦闪点,磷坦姆沙韦溶解度,磷坦姆沙韦旋光度,磷坦姆沙韦MSDS,磷坦姆沙韦熔点,
| 产品说明 | 磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)是BMS-626529的原药,能结合gp120,抑制HIV-1与细胞CD4受体的结合。 |
| Introduction | 磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)is the phosphonooxymethyl prodrug of BMS26529. Fostemsavir is a novel attachment inhibitor that targetsHIVgp120 and prevents its binding to CD4+T cells. |
| Application1 | BMS-626529(一种HIV-1附着抑制剂)的前药。 |
| Application2 | Prodrug of BMS-626529, an HIV-1 attachment inhibitor. |
| Application3 |
磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)药理学:
※BMS-663068是开发用于治疗HIV-1感染的HIV-1附着抑制剂。; IC50值:;目标:HIVBMS-663068是BMS-626529的前药,它与病毒包膜糖蛋白gp120结合并干扰病毒与细胞CD4受体的附着。在有或没有利托那韦的情况下,连续8天使用BMS-663068会导致血浆HIV-1 RNA水平大幅下降,并且通常具有良好的耐受性。BMS-663068作为抗逆转录病毒疗法联合治疗的一部分,需要进行长期的临床试验。
※Fostemsavir(BMS-663068)是一种实验性HIV进入抑制剂,是temsavir(BMS-626529)的前药,用于治疗HIV感染。 通过阻断病毒的gp120受体,它可以防止病毒最初附着于宿主CD4 + T细胞并进入宿主免疫细胞。 它的作用方法是第一个针对艾滋病毒的药物。 因为它针对病毒生命周期的不同步骤,所以它为具有对其他HIV药物高度耐药性的病毒个体提供了希望。 由于gp120是病毒的高度保守区域,该药物不太可能通过产生CD4无关病毒来增强自身抗药性。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Nowicka-Sans B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012 Jul;56(7):3498-507 |
| Effects of Temsavir, Active Moiety of Antiretroviral Agent Fostemsavir, on QT Interval: Results From a Phase I Study and an Exposure-Response Analysis PMID 32027457; Clinical and translational science |
| Model-Based Phase 3 Dose Selection for HIV-1 Attachment Inhibitor Prodrug BMS-663068 in HIV-1-Infected Patients: Population Pharmacokinetics/Pharmacodynamics of the Active Moiety, BMS-626529 PMID 2690 |
| Inhibitors of HIV-1 Attachment: The Discovery and Development of Temsavir and its Prodrug Fostemsavir PMID 29271653; Journal of medicinal chemistry 2018 01; 61(1):62-80 Name matches: temsavir fostemsa |
| Pharmacokinetic interactions between BMS-626529, the active moiety of the HIV-1 attachment inhibitor prodrug BMS-663068, and ritonavir or ritonavir-boosted atazanavir in healthy subjects PMID 25870057 |
磷坦姆沙韦(864953-29-7,BMS-663068,Fostemsavir)参考文献:
1.Model-Based Phase 3 Dose Selection for HIV-1 Attachment Inhibitor Prodrug BMS-663068 in HIV-1-Infected Patients: Population Pharmacokinetics/Pharmacodynamics of the Active Moiety, BMS-626529.
Landry I1, Zhu L1, Abu Tarif M1, Hruska M1, Sadler BM2, Pitsiu M3, Joshi S4, Hanna GJ1, Lataillade M4, Boulton DW1, Bertz RJ5. Antimicrob Agents Chemother. 2016 Apr 22;60(5):2782-9. doi: 10.1128/AAC.02503-15. Print 2016 May.
BMS-663068 is an oral prodrug of the HIV-1 attachment inhibitor BMS-626529, which prevents viral attachment to host CD4(+) T cells by binding to HIV-1 gp120. To guide dose selection for the phase 3 program, pharmacokinetic/pharmacodynamic modeling was performed using data from two phase 2 studies with HIV-1-infected subjects (n = 244). BMS-626529 population pharmacokinetics were described by a two-compartment model with first-order elimination from the central compartment, zero-order release of prodrug from the extended-release formulation into a hypothetical absorption compartment, and first-order absorption into the central compartment. The covariates of BMS-663068 formulation type, lean body mass, baseline CD8(+) T-cell percentage, and ritonavir coadministration were found to be significant contributors to intersubject variability. Exposure-response analyses showed a relationship between the loge-transformed concentration at the end of a dosing interval (Ctau) normalized for the protein binding-adjusted BMS-626529 half-maximal (50%) inhibitory concentration (PBAIC50) and the change in the HIV-1 RNA level from the baseline level after 7 days of BMS-663068 monotherapy.
2.Pharmacokinetic interactions between BMS-626529, the active moiety of the HIV-1 attachment inhibitor prodrug BMS-663068, and ritonavir or ritonavir-boosted atazanavir in healthy subjects.
Zhu L1, Hruska M2, Hwang C2, Shah V2, Furlong M2, Hanna GJ2, Bertz R2, Landry IS2. Antimicrob Agents Chemother. 2015 Jul;59(7):3816-22. doi: 10.1128/AAC.04914-14. Epub 2015 Apr 13.
BMS-663068 is a prodrug of BMS-626529, a first-in-class attachment inhibitor that binds directly to HIV-1 gp120, preventing initial viral attachment and entry into host CD4(+) T cells. This open-label, multiple-dose, four-sequence, crossover study addressed potential two-way drug-drug interactions following coadministration of BMS-663068 (BMS-626529 is a CYP3A4 substrate), atazanavir (ATV), and ritonavir (RTV) (ATV and RTV are CYP3A4 inhibitors). Thirty-six healthy subjects were randomized 1:1:1:1 to receive one of four treatment sequences with three consecutive treatments: BMS-663068 at 600 mg twice daily (BID), BMS-663068 at 600 mg BID plus RTV at 100 mg once daily (QD), ATV at 300 mg QD plus RTV at 100 mg QD (RTV-boosted ATV [ATV/r]), or BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD. Compared with the results obtained by administration of BMS-663068 alone, coadministration of BMS-663068 with ATV/r increased the BMS-626529 maximum concentration in plasma (Cmax) and the area under the concentration-time curve in one dosing interval (AUCtau) by 68% and 54%, respectively.
3.Safety and efficacy of the HIV-1 attachment inhibitor prodrug BMS-663068 in treatment-experienced individuals: 24 week results of AI438011, a phase 2b, randomised controlled trial.
Lalezari JP1, Latiff GH2, Brinson C3, Echevarría J4, Treviño-Pérez S5, Bogner JR6, Thompson M7, Fourie J8, Sussmann Pena OA9, Mendo Urbina FC10, Martins M11, Diaconescu IG12, Stock DA13, Joshi SR13, Hanna GJ14, Lataillade M13; AI438011 study team. Lancet HIV. 2015 Oct;2(10):e427-37. doi: 10.1016/S2352-3018(15)00177-0. Epub 2015 Sep 1.
BACKGROUND: BMS-663068 is an oral prodrug of BMS-626529, an attachment inhibitor that binds to HIV-1 gp120, blocking viral attachment to host CD4 cells. AI438011 is an ongoing trial investigating the efficacy, safety, and dose-response of BMS-663068 in treatment-experienced, HIV-1-infected patients. Herein we present the results of the primary analysis.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



