-
多拉韦林
- names:
Doravirine
- CAS号:
1338225-97-0
MDL Number: MFCD22417167 - MF(分子式): C17H11ClF3N5O3 MW(分子量): 425.75
- EINECS: Reaxys Number:
- Pubchem ID:58460047 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000623-5mg | 5mg | >98.0% | ¥ 2430.00 | ¥ 2430.00 | 2-3天 | ¥ 0.00 | ||
| YZM000623-2mg | 2mg | >98.0% | ¥ 1365.00 | ¥ 1365.00 | 2-3天 | ¥ 0.00 |
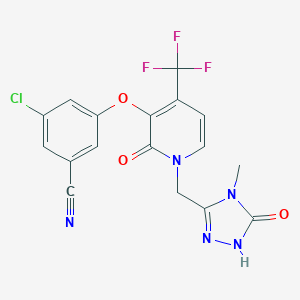
| 中文别名 | 多拉韦林(1338225-97-0),MK-1439,皮菲特罗,3-氯-5-((1-((4-甲基-5-氧代-4,5-二氢-1H-1,2,4-三唑-3-基)甲基)-2 -氧代-4-(三氟甲基)-1,2-二氢吡啶-3-基氧基)苄腈; |
| 英文别名 | Doravirine(1338225-97-0),DORAVIRINE,MK-1439,Pifeltro,3-Chloro-5-((1-((4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl)-2-oxo-4-(trifluoromethyl)-1,2-dihydropyridin-3-yl)oxy)benzonitrile; |
| CAS号 | 1338225-97-0 |
| Inchi | InChI = 1S / C17H11ClF3N5O3 / c1-25-13(23-24-16(25)28)8-26-3-2-12(17(19,20)21)14(15(26)27)29- 11-5-9(7-22)4-10(18)6-11 / h2-6H,8H2,1H3,(H,24,28) |
| InchiKey | ZIAOVIPSKUPPQW-UHFFFAOYSA-N |
| 分子式 Formula | C17H11ClF3N5O3 |
| 分子量 Molecular Weight | 425.75 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度≥ 30 mg/mL(70.46 mM)*"≥" means soluble可溶, but saturation unknown溶解度未知. |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C冰柜 |
多拉韦林,Doravirine(1338225-97-0,MK-1439)药理学:
在一项临床2期试验中,在没有抗逆转录病毒治疗史的HIV-1感染者中,剂量范围为0.25-2倍推荐剂量的多拉韦林(与恩曲他滨 / 替诺福韦联合使用),未发现疗效与暴露-应答关系[L12729]此外,以1200 mg的剂量提供推荐剂量后观察到的峰值浓度的约4倍,多拉韦林不会将QT间隔延长至任何临床相关程度。
多拉韦林是一种非核苷类逆转录酶抑制剂,可与其他抗逆转录病毒药物联合用于治疗人类免疫缺陷病毒(HIV)感染。多拉韦林与治疗期间短暂的血清氨基转移酶升高率低相关,但与临床上明显的急性肝损伤无关。
多拉韦林是一种HIV-1非核苷类逆转录酶抑制剂(NNRTI),旨在与其他抗逆转录病毒药物联用。多拉韦林可以单独使用,也可以作为多拉韦林(100 mg),拉米夫定(300 mg)和替诺福韦二甲酚富马酸盐(300 mg)的组合产品获得。多拉韦林正式用于没有抗逆转录病毒治疗经验的成年患者中治疗HIV-1感染,进一步扩大了可用于治疗HIV-1感染的治疗方法的可能性和选择。
多拉韦林是一种非核苷类逆转录酶抑制剂。体外IC 50值为野生型12 nM,针对K103N的21 nM,针对Y181C的31 nM,针对K103N / Y181C突变病毒的33 nM。MK-1439对10种不同的HIV-1亚型病毒表现出相似的抗病毒活性。治疗HIV-1感染的第三阶段正在进行中。
多拉韦林,Doravirine(1338225-97-0,MK-1439)毒理性质:
据报道,在接受多拉韦林治疗的患者中,有13%的患者血清氨基转移酶升高,但在正常水平上限的5倍以上时升高并不常见,发生在1%或更少的患者中。在并发乙型或丙型肝炎的患者中,多拉韦林治疗期间血清氨基转移酶升高的比率较高,但异常情况很少严重。多拉韦林仅在临床上使用了很短的时间,但是与许多其他非核苷类逆转录酶抑制剂不同,多拉韦林尚未与临床上明显的肝损伤报道有关。 可能性评分:E *(可疑但未经证实的临床上明显的肝损伤原因)。
多拉韦林,Doravirine(1338225-97-0,MK-1439)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:多拉韦林试剂,多拉韦林杂质,多拉韦林中间体,多拉韦林密度,多拉韦林溶解度,多拉韦林旋光度,多拉韦林闪点,多拉韦林熔点,多拉韦林购买,
| 产品说明 | (1338225-97-0,Doravirine,MK-1439)多拉韦林是一种有效的高度特异性的HIV-1非核苷逆转录酶的抑制剂,对野生型以及K103N和Y181C逆转录酶突变体的IC50值分别是 4.5 nM,5.5 nM 和 6.1 nM |
| Introduction | Doravirine (MK439,多拉韦林,1338225-97-0)is a highly specificHIV nonnucleoside reverse transcriptaseinhibitor withIC50s of4.5nM, 5.5nM and 6. |
| Application1 | 1nM against the wild type andK103NandY181Creverse transcriptasemutants, respectively. |
| Application2 | |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Lai MT et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014; |
| Anderson MS et al. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Th |
1,单次和多次服用利福平对健康受试者中Doravirine药代动力学的影响。
Yee KL; Khalilieh SG; Sanchez RI; Liu R; Anderson MS; Manthos H;法官T; Brejda J; Butterton JR临床药物研究。2017年7月; 37(7):659-667。doi:10.1007 / s40261-017-0513-4。
BACKGROUND AND OBJECTIVE: ;Doravirine is a novel, next-generation, non-nucleoside reverse transcriptase inhibitor in development for the treatment of human immunodeficiency virus-1 infection in combination with other antiretrovirals. Doravirine is a substrate for cytochrome P450 (CYP) 3A and P-glycoprotein. Rifampin (rifampicin) is used for treating tuberculosis in patients who are co-infected with human immunodeficiency virus. Rifampin demonstrates organic anion-transporting polypeptide 1B1 and P-glycoprotein inhibition after single-dose administration and CYP3A and P-glycoprotein induction after multiple-dose administration. The objective of this study was to evaluate the effects of co-administration of single and multiple doses of rifampin on doravirine pharmacokinetics.;METHODS: ;In period 1 of this open-label, two-period, fixed-sequence study in healthy adults, subjects received single-dose doravirine 100 mg; blood samples for measuring plasma concentration were collected pre-dose and up to 72 h post-dose. In period 2, following a 7-day washout, subjects received doravirine 100 mg and rifampin 600 mg on day 1, rifampin 600 mg daily on days 4-18, with doravirine 100 mg co-administered on day 17; blood samples were collected pre-dose and up to 72 h post-dose on day 1 and up to 48 h post-dose on day 17.
2.Doravirine:评论。
马萨诸塞州的库隆比尔(Colombier MA);莫琳娜(J)2018年7月; 13(4):308-314。doi:10.1097 / COH.0000000000000471。
Colombier MA;Molina JM Curr Opin HIV AIDS. 2018 Jul;13(4):308-314. doi: 10.1097/COH.0000000000000471.
PURPOSE OF REVIEW: ;The current review addresses the role of doravirine (DOR), a novel once-daily nonnucleoside reverse transcriptase inhibitor (NNRTI) in first-line therapy at a time in which multiple options are available, and issues of antiviral efficacy, safety, simplicity and cost are critical to make informed decisions.;RECENT FINDINGS: ;DOR combination regimens have been tested in two large randomized double-blinded clinical trials in treatment-naïve patients, showing noninferiority to ritonavir-boosted darunavir-based and efavirenz (EFV)-based regimens. The main features of DOR are reviewed in this report including its antiviral activity, genetic barrier to resistance, safety, once-daily dosing and coformulation in a single tablet with tenofovir disoproxil fumarate and lamivudine. DOR pharmacokinetics and drug-drug interactions are also reviewed as DOR can be given without food restriction and has no interaction with proton pump inhibitors. DOR has shown a superior safety profile than EFV regarding neuropsychiatric and cutaneous adverse events. DOR is currently being investigated in treatment-experienced patients and in those with transmitted NNRTI drug resistance.;SUMMARY: ;DOR is a promising new NNRTI that could become the preferred drug in its class for treatment initiation.
3,2020年艾滋病治疗:会是什么样?
Gulick RJ国际艾滋病协会。2014年11月2日; 17(4增刊3):19528。doi:10.7448 / IAS.17.4.19528。eCollection 2014。
Currently there are 28 approved antiretroviral drugs in six mechanistic classes, and recommended first-line regimens are highly potent, well tolerated, and as convenient as one pill, once-a-day. How will HIV treatment change by 2020? Over the next few years, we are likely to see potent 2-drug regimens tested head-to-head with standard three-drug regimens, and some of these will likely become standard-of-care. Newer agents with novel drug resistance profiles (e.g. doravirine, an NNRTI) or new mechanisms of action (e.g. BMS 663068, a CD4 attachment inhibitor) will provide virologic activity in patients with drug-resistant viral strains. Comparative studies of current and newer agents such as the investigational prodrug of tenofovir (TAF) will help define less toxic regimens. We will see additional convenient co-formulations developed; with them, we are likely to have second- and even third-line regimens administered one pill, once-daily. Long-acting injectable investigational formulations currently in clinical trials such as rilpivirine LA (administered monthly) and cabotegravir (administered quarterly), and others (including combinations of these agents) could provide additional convenient treatment options.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



