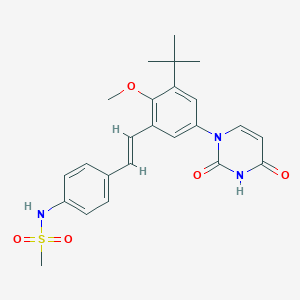
-
ABT-072
- names:
ABT-072
- CAS号:
1132936-00-5
MDL Number: MFCD28502074 - MF(分子式): C24H27N3O5S MW(分子量): 469.55
- EINECS: Reaxys Number:
- Pubchem ID:57775240 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000581-5mg | 5mg | >99.0% | ¥ 5568.00 | ¥ 5568.00 | Backorder | ¥ 0.00 | ||
| YZM000581-1mg | 1mg | >99.0% | ¥ 2730.00 | ¥ 2730.00 | 2-3天 | ¥ 0.00 |
| 中文别名 | ABT-072(cas:1132936-00-5);ABT072;ABT 072; |
| 英文别名 | ABT-072(cas:1132936-00-5);ABT072;ABT 072;(E)-N-(4-(3-(tert-Butyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxystyryl)phenyl)methanesulfonamide |
| CAS号 | 1132936-00-5 |
| Inchi | InChI=1S/C24H27N3O5S/c1-24(2,3)20-15-19(27-13-12-21(28)25-23(27)29)14-17(22(20)32-4)9-6-16-7-10-18(11-8-16)26-33(5,30)31/h6-15,26H,1-5H3,(H,25,28,29)/b9-6+ |
| InchiKey | XMZSTQYSBYEENY-RMKNXTFCSA-N |
| 分子式 Formula | C24H27N3O5S |
| 分子量 Molecular Weight | 469.55 |
| 溶解度Solubility | |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:ABT-072蒸汽压,ABT-072合成,ABT-072标准,ABT-072应用,ABT-072合成,ABT-072沸点,ABT-072闪点,ABT-072用途,ABT-072溶解度,ABT-072价格,ABT-072作用,ABT-072结构式,ABT-072用处
| 产品说明 | ABT-072(1132936-00-5)是非核苷NS5B polymerase的抑制剂,是治疗HCV的有效候选药 |
| Introduction | ABT72 (1132936-00-5)is a nonnucleosideNS5B polymeraseinhibitor and a candidate drug evaluated for treatment of hepatitis C virus. |
| Application1 | |
| Application2 | |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| A Randomized Study to Evaluate the Safety, Tolerability and Antiviral Activity of ABT-450, ABT-333 and ABT-072 Phase 2 Completed 2015-01-08 |
| A Pilot Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Antiviral Activity of ABT-450 With Ritonavir (ABT-450/r) Dosed in Combination With ABT-072 and Ribavirin (RBV) Phase 2 Comple |
| A Follow-up Study to Assess Resistance to ABT-072 in HCV-infected Subjects Administered ABT-072 in Prior ABT-072 Studies Phase 2 Completed 2013-04-01 |
| A Study of Single Doses of ABT-072 in Japanese Healthy Male Adults Phase 1 Completed 2010-10-25 |
| A Study of ABT-072 in Healthy and Hepatitis C Virus Genotype 1-Infected Adults Phase 1 Completed 2010-10-21 |
Abstract:
Background & aims: ABT-450 (combined with low-dose ritonavir, ABT-450/r) is a potent HCV NS3 protease inhibitor, and ABT-072 is a non-nucleoside NS5B polymerase inhibitor. The goal of this study was to evaluate the safety, tolerability, and efficacy of the peginterferon-free combination of ABT-450/r and ABT-072 with ribavirin in treatment-naïve patients with IL28B CC genotype, infected with HCV genotype 1.
Methods: This was a phase 2a, multicenter, open-label, single-arm study in 11 treatment-naïve, non-cirrhotic HCV GT1-infected patients with IL28B rs12979860 genotype CC. Patients received ABT-450/r 150/100 mg once daily and ABT-072 400 mg once daily with weight-based ribavirin 1000-1200 mg/day dosed twice daily for 12 weeks.
Results: Eight (73%) patients were male, 9 (82%) were Caucasian (including 3 who self-identified as Hispanic); mean baseline HCV RNA was 6.9 log?? IU/ml (range 6.5-7.3 log?? IU/ml). All 11 patients completed 12 weeks of treatment and maintained HCV RNA <25 IU/ml from weeks 4 through 12 of treatment. Ten patients (91%) achieved sustained virologic response 24 weeks post-treatment, with a second patient relapsing 36 weeks post-treatment. There were no deaths, serious or severe adverse events, or premature discontinuations. Adverse events were mostly mild and the most frequent were headache, fatigue, nausea, and dry skin.
Conclusions: A 12-week regimen of ABT-450/r and ABT-072 with ribavirin was well tolerated with 9/11 patients achieving sustained virologic response through 36 weeks of post-treatment observation. These findings suggest that peginterferon-free regimens may have the potential to cure a high proportion of HCV genotype 1-infected patients.
2.Current prospects for interferon-free treatment of hepatitis C in 2012/PMID 23137126; Journal of gastroenterology and hepatology 2013 Jan; 28(1):38-45 (Review Article)/Name matches: abt-450 abt-072
Abstract:Present interferon-based therapy for chronic hepatitis C is limited by both efficacy and tolerability. Telaprevir and boceprevir are the first two direct-acting antiviral drugs (DAAs) that inhibit hepatitis C virus replication to be licensed for use in conjunction with pegylated interferon and ribavirin. Numerous other DAAs are in clinical development, and phases 2 and 3 trials are evaluating interferon-free combination DAA therapy. Interferon-free sustained virologic responses have now been achieved with combinations of asunaprevir and daclatasvir; sofosbuvir and ribavirin; sofosbuvir and daclatasvir; faldaprevir and BI207127; ABT-450, ritonovir and ABT-333; ABT-450, ritonovir and ABT-072; miracitabine, danoprevir and ritonavir; and alisporivir and ribavirin. Some drugs are genotype-specific in their activity, whereas others are pan-genotypic, and differential responses for the genotype 1 subtypes 1a and 1b have emerged with many DAA combinations. Viral breakthrough and resistance are important considerations for future trial design. The prospect of interferon-free combination DAA therapy for hepatitis C virus is now finally becoming a reality.
3.Synthesis and Biological Characterization of Aryl Uracil Inhibitors of Hepatitis C Virus NS5B Polymerase: Discovery of ABT-072, a trans-Stilbene Analog with Good Oral Bioavailability/PMID 29342358; Journal of medicinal chemistry 2018 02; 61(3):1153-1163/Name matches: abt-450 abt-072
Abstract:ABT-072 is a non-nucleoside HCV NS5B polymerase inhibitor that was discovered as part of a program to identify new direct-acting antivirals (DAAs) for the treatment of HCV infection. This compound was identified during a medicinal chemistry effort to improve on an original lead, inhibitor 1, which we described in a previous publication. Replacement of the amide linkage in 1 with a trans-olefin resulted in improved compound permeability and solubility and provided much better pharmacokinetic properties in preclinical species. Replacement of the dihydrouracil in 1 with an N-linked uracil provided better potency in the genotype 1 replicon assay. Results from phase 1 clinical studies supported once-daily oral dosing with ABT-072 in HCV infected patients. A phase 2 clinical study that combined ABT-072 with the HCV protease inhibitor ABT-450 provided a sustained virologic response at 24 weeks after dosing (SVR24) in 10 of 11 patients who received treatment.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
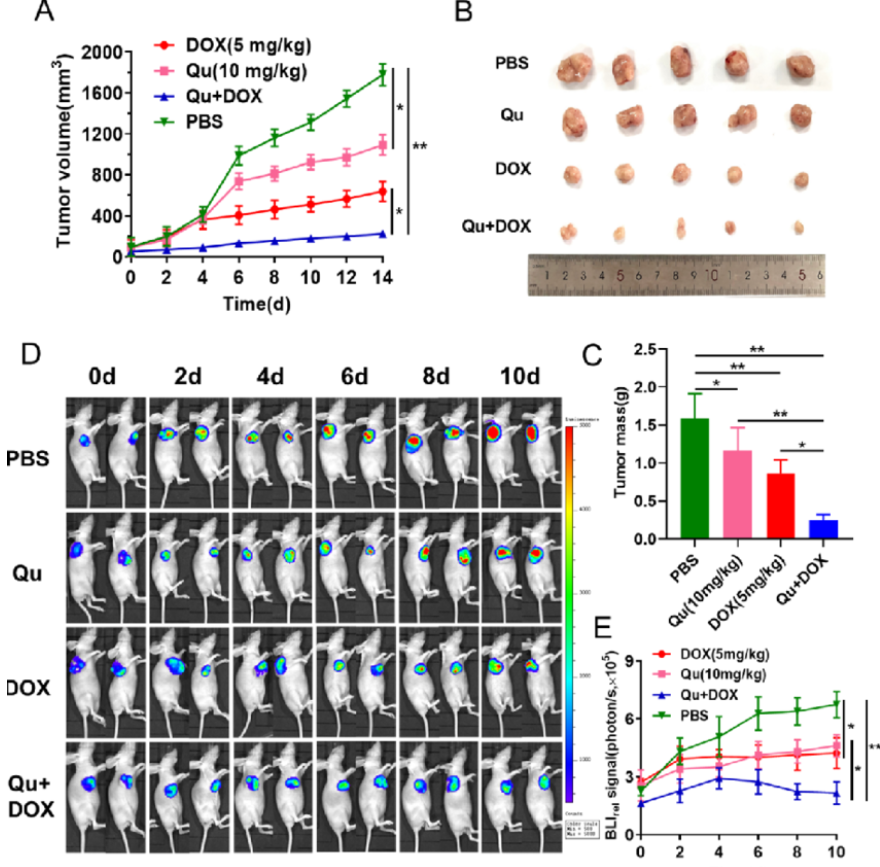
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



