-
雷迪帕韦
- names:
Ledipasvir
- CAS号:
1256388-51-8
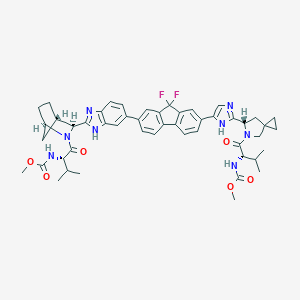
MDL Number: MFCD25976756 - MF(分子式): C49H54F2N8O6 MW(分子量): 889
- EINECS: Reaxys Number:
- Pubchem ID:67505836 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000549B-5mg | 5mg | 99.96% | ¥ 1215.00 | ¥ 1215.00 | 2-3天 | ¥ 0.00 | ||
| YZM000549B-2mg | 1mg | 99.96% | ¥ 620.00 | ¥ 620.00 | Instock1-2days | ¥ 0.00 |
| 中文别名 | 雷迪帕韦(cas:1256388-51-8),雷迪帕维,雷地帕韦,GS-5885,GS5885,GS 5885;丙肝病毒NS5A聚合酶抑制剂 |
| 英文别名 | Ledipasvir(cas:1256388-51-8),GS-5885,GS5885,GS 5885 |
| CAS号 | 1256388-51-8 |
| Inchi | InChI=1S/C49H54F2N8O6/c1-24(2)39(56-46(62)64-5)44(60)58-23-48(15-16-48)21-38(58)42-52-22-37(55-42)28-9-13-32-31-12-8-26(18-33(31)49(50,51)34(32)19-28)27-10-14-35-36(20-27)54-43(53-35)41-29-7-11-30(17-29)59(41)45(61)40(25(3)4)57-47(63)65-6/h8-10,12-14,18-20,22,24-25,29-30,38-41H,7,11,15-17,21,23H2,1-6H3,(H,52,55)(H,53,54)(H,56,62)(H,57,63)/t29-,30+,38-,39-,40-,41-/m0/s |
| InchiKey | VRTWBAAJJOHBQU-KMWAZVGDSA-N |
| 分子式 Formula | C49H54F2N8O6 |
| 分子量 Molecular Weight | 889 |
| 溶解度Solubility | 生物体外In Vitro:DMSO溶解度50 mg/mL(56.24 mM;Need ultrasonic)H2O< 0.1 mg/mL(insoluble) |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:雷迪帕韦蒸汽压,雷迪帕韦合成,雷迪帕韦标准,雷迪帕韦应用,雷迪帕韦合成,雷迪帕韦沸点,雷迪帕韦闪点,雷迪帕韦用途,雷迪帕韦溶解度,雷迪帕韦价格,雷迪帕韦作用,雷迪帕韦结构式,雷迪帕韦用处,雷迪帕韦毒理性质
| 产品说明 | 雷迪帕韦(Ledipasvir,1256388-51-8)是一种有效的HCV NS5A的抑制剂, |
| Introduction | Ledipasvir(雷迪帕韦,1256388-51-8) is an inhibitor of thehepatitis C virus NS5A, withEC50s of 34 pM and 4 pM against genotype 1a and 1b replicon, respectively. |
| Application1 | 能够抑制 GT1a 和 GT1b 复制子,EC50值分别是 34 pM 和 4 pM |
| Application2 | |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19 bioRxiv : the preprint server for biology 2020-06-23 |
| Drug repurposing using computational methods to identify therapeutic options for COVID-19 Journal of diabetes and metabolic disorders 2020-05-30 |
| Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates F1000Research 2020-01-01 |
| Ledipasvir Pharmaceutical Substances 2015 |
| Ledipasvir/sofosbuvir in chronic hepatitis C: a guide to its use in the EU Drugs & Therapy Perspectives 2015 |
Abstract:A single-tablet regimen of ledipasvir and sofosbuvir (ledipasvir/sofosbuvir; Harvoni®) was recently approved in the EU. The phase 3 ION trials included treatment-naïve (ION-1 and -3) or treatment-experienced (ION-2) patients with chronic hepatitis C virus (HCV) genotype 1 infection; ≈20% of patients in ION-1 and -2 had cirrhosis, whereas none of the patients in ION-3 had cirrhosis. In ION-1, a 12-week regimen of ledipasvir/sofosbuvir achieved high rates of sustained virological response 12 weeks' post-treatment (SVR12) in treatment-naïve patients, with no additional benefit conferred by the addition of ribavirin or extending the treatment duration to 24 weeks. An 8-week regimen also achieved high SVR12 rates in patients with a baseline HCV RNA level of <6 million IU/mL in ION-3. High SVR12 rates were seen in treatment-experienced patients who received ledipasvir/sofosbuvir for 12 or 24 weeks in ION-2. Data also support the use of ledipasvir/sofosbuvir in chronic HCV genotype 4 infecti on, in HCV and HIV co-infection and, in combination with ribavirin, in patients with chronic HCV genotype 1 or 4 infection who have decompensated cirrhosis or are liver transplant recipients and in chronic HCV genotype 3 infection. Oral ledipasvir/sofosbuvir was generally well tolerated.
2.Drug–Drug Interaction Profile of the Fixed-Dose Combination Tablet Regimen Ledipasvir/Sofosbuvir Clinical Pharmacokinetics 2018
Abstract:Ledipasvir/sofosbuvir (Harvoni®), a fixed-dose combination tablet of an NS5A inhibitor ledipasvir and an NS5B polymerase inhibitor sofosbuvir, is approved for the treatment of chronic hepatitis C virus infection. Ledipasvir/sofosbuvir exhibits a favorable drug--drug interaction profile and can be administered with various medications that may be used by hepatitis C virus-infected patients, including patients with comorbidities, such as co-infection with human immunodeficiency virus or immunosuppression following liver transplantation. Ledipasvir/sofosbuvir is not expected to act as a victim or perpetrator of cytochrome P450- or UDP-glucuronosyltransferase 1A1-mediated drug--drug interactions. With the exception of strong inducers of P-glycoprotein, such as rifampin, ledipasvir/sofosbuvir is not expected to act as a victim of clinically relevant drug--drug interactions . As a perpetrator of pharmacokinetic drug–drug interactions via P-glycoprotein/BCRP, ledipasvir/sofosbuvir should not be u sed with rosuvastatin and elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate, whereas its co-administration with amiodarone is not recommended because of a pharmacodynamic interaction. This review summarizes a number of drug interaction studies conducted in support of the clinical development of ledipasvir/sofosbuvir .
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



