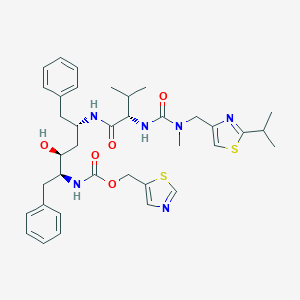
-
利托那韦
- names:
Ritonavir
- CAS号:
155213-67-5
MDL Number: - MF(分子式): C37H48N6O5S2 MW(分子量): 720.3127601
- EINECS: Reaxys Number:
- Pubchem ID:392622 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| HCQ000017-250mg | 250mg | 99% | ¥ 2150.00 | ¥ 2150.00 | 1-2days Instock | ¥ 0.00 | ||
| HCQ000017-100mg | 100mg | 99% | ¥ 1420.00 | ¥ 1420.00 | 1-2days Instock | ¥ 0.00 | ||
| HCQ000017-25mg | 25mg | 99% | ¥ 480.00 | ¥ 480.00 | 1-2days Instock | ¥ 0.00 |
| 中文别名 | 瑞托那韦; 蛋白酶抑制剂; 利托那韦(cas,msds,应用) |
| 英文别名 | Ritonavir(155213-67-5) |
| CAS号 | 155213-67-5 |
| Inchi | 1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 |
| InchiKey | NCDNCNXCDXHOMX-XGKFQTDJSA-N |
| 分子式 Formula | C37H48N6O5S2 |
| 分子量 Molecular Weight | 720.3127601 |
| 溶解度Solubility | DMSO: soluble10mg/mL |
| 性状 | 固体粉末 |
| 储藏条件 Storage conditions | -20°C,-4℃存储 |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of resveratrol used for a mouse (22.4 mg/kg) to a dose based on the BSA for a rat, multiply 22.4 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for resveratrol of 11.2 mg/kg.
| 产品说明 | 利托那韦(CAS:155213-67-5,英文名:Ritonavir)属于蛋白酶抑制剂类的抗逆转录病毒小分子化合物,利托那韦可用于治疗HIV感染以及艾滋病。 |
| Introduction | Ritonavir is an HIV protease inhibitor that interferes with the reproductive cycle of HIV. Although it was initially developed as an independent antiviral agent, it has been shown to possess advantageous properties in combination regimens with low-dose ritonavir and other protease inhibitors. It is now more commonly used as a booster of other protease inhibitors and is available in both liquid formulation and as capsules. While ritonavir is not an active antiviral agent against hepatitis C virus (HCV) infection, it is added in combination therapies indicated for treatment of HCV infections as a booster. Ritonavir is a potent CYP3A inhibitor that increases peak and trough plasma drug concentrations of other protease inhibitors such as Paritaprevir and overall drug exposure. American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) guidelines recommend ritonavir-boosted combination therapies as a first-line therapy for HCV Genotype 1a/b and 4 treatment-na?ve patients with or without cirrhosis. Ritonavir is found in a fixed-dose combination product with Ombitasvir, Dasabuvir, and Paritaprevir as the FDA-approved product Viekira Pak. First approved in December 2014, Viekira Pak is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis.Ritonavir is also available as a fixed-dose combination product with Ombitasvir and Paritaprevir as the FDA- and Health Canada-approved product Technivie. First approved in July 2015, Technivie is indicated in combination with Ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis.In Canada, ritonavir is also available as a fixed-dose combination product with Ombitasvir, Dasabuvir, and Paritaprevir as the Health Canada-approved, commercially available product Holkira Pak. First approved in January 2015, Holkira Pak is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a with or without cirrhosis. Inclusion of ritonavir can can select for HIV-1 protease inhibitor resistance-associated substitutions. Any HCV/HIV-1 co-infected patients treated with ritonavir-containing combination therapies should also be on a suppressive antiretroviral drug regimen to reduce the risk of HIV-1 protease inhibitor drug resistance. |
| Application1 | 潜在冠状病毒抑制剂Experimental Unapproved Treatments for COVID-19 |
| Application2 | |
| Application3 |
Ritonavir 是一种蛋白酶抑制剂类抗逆转录病毒化合物,用于治疗HIV感染和艾滋病。利托那韦(ABT-538)诱导CYP 1A2并抑制主要的P450亚型(3A4和2D6)。 HAART经常与利托那韦一起开处方,并不是因为它具有抗病毒作用,而是因为它抑制与其他蛋白酶抑制剂代谢相同的宿主酶,从而使临床医生能够降低其剂量和频率并提高其临床疗效。更具体地说,利托那韦(ABT-538)用于抑制通常代谢蛋白酶抑制剂细胞色素P450-3A4(CYP3A4)的特定肝酶。利托那韦对细胞色素P-450 CYP3A4酶的抑制作用会降低同时给药的蛋白酶抑制剂的代谢并改变其药代动力学参数,包括曲线下面积(AUC),最大浓度(Cmax),最小浓度(Cmin)和半衰期(t1) / 2)。结果,增强的蛋白酶抑制剂的生物利用度得以提高,并且可以提高对HIV贮库的渗透。
| Hull MW, Montaner JS: Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011 Aug;43(5):375-88. doi: 10.3109/07853890.2011.572905. Epub 2011 Apr 18. [PubMed:21501034] |
| Myers RP, Shah H, Burak KW, Cooper C, Feld JJ: An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol Hepatol. 2015 Jan-Feb;29(1):19-34. Epub 2015 Jan 13. [PubMed:25585348] |
| Sevrioukova IF, Poulos TL: Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A. 2010 Oct 26;107(43):18422-7. doi: 10.1073/pnas.1010693107. Epub 2010 Oct 11. [PubMed:20937904] |
| Rock BM, Hengel SM, Rock DA, Wienkers LC, Kunze KL: Characterization of ritonavir-mediated inactivation of cytochrome P450 3A4. Mol Pharmacol. 2014 Dec;86(6):665-74. doi: 10.1124/mol.114.094862. Epub 2014 Oct 1. [PubMed:25274602] |
| Tseng A, Hughes CA, Wu J, Seet J, Phillips EJ: Cobicistat Versus Ritonavir: Similar Pharmacokinetic Enhancers But Some Important Differences. Ann Pharmacother. 2017 Nov;51(11):1008-1022. doi: 10.1177/1060028017717018. Epub 2017 Jun 19. [PubMed:28627229] |
FDA Approved Drug Products: NORVIR (ritonavir) Capsules, Soft Gelatin for Oral use [Link]
FDA Approved Drug Products: Norvir (ritonavir) for oral use [Link]
CaymanChem: Ritonavir MSDS [Link]
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



