-
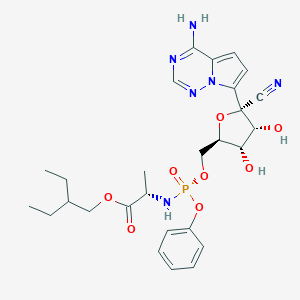
瑞德西韦
- names:
Remdesivir(Synonyms:GS-5734)
- CAS号:
1809249-37-3
MDL Number: - MF(分子式): C27H35N6O8P MW(分子量): 602.2253991
- EINECS: Reaxys Number:
- Pubchem ID:121304016 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| HCY00005-5mg | 5mg | 98% | ¥ 1800.00 | ¥ 1800.00 | instock | ¥ 0.00 | ||
| HCY00005-10mg | 10mg | 98% | ¥ 2800.00 | ¥ 2800.00 | instock | ¥ 0.00 | ||
| HCY00005-50mg | 50mg | 98% | ¥ 5800.00 | ¥ 5800.00 | instock | ¥ 0.00 | ||
| HCY00005-1g | 1g | 98% | ¥ 13800.00 | ¥ 13800.00 | instock | ¥ 0.00 |
| 中文别名 | 瑞德沙韦,瑞德西韦(1809249-37-3,GS-5734),瑞德西韦磷酸盐,瑞德西韦异构体, |
| 英文别名 | Remdesivir,GS-5734,GS 5734,GS5734,1809249-37-3, |
| CAS号 | 1809249-37-3 |
| Inchi | InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 |
| InchiKey | NCDNCNXCDXHOMX-XGKFQTDJSA-N |
| 分子式 Formula | C27H35N6O8P |
| 分子量 Molecular Weight | 602.2253991 |
| 溶解度Solubility | |
| 性状 | 固体粉末,Powder |
| 储藏条件 Storage conditions | 阴凉干燥处处;Storge at room temperature |
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of resveratrol used for a mouse (22.4 mg/kg) to a dose based on the BSA for a rat, multiply 22.4 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for resveratrol of 11.2 mg/kg.
Tags:瑞德西韦供应商,Remdesivir,瑞德西韦一磷酸,瑞德西韦二磷酸,瑞德西韦三磷酸,瑞德西韦购买,Remdesivir 瑞德西韦生产,Remdesivir 瑞德西韦 批量,Remdesivir 瑞德西韦 供应,Remdesivir 瑞德西韦 订购,Remdesivir 瑞德西韦采购,瑞德西韦厂家,GS-5734,GS 5734,GS5734,1809249-37-3,Remdesivir,瑞德西韦合成,瑞德西韦的作用,Remdesivir合成原料,Remdesivir中间体

| 产品说明 | Remdesivir(1809249-37-3,GS-5734;Remdesivir)是一种病毒抑制剂,Remdesivir(GS-5734)已经衍生出瑞德西韦一磷酸,瑞德西韦二磷酸,瑞德西韦三磷酸(Remdesivir triphosphate)等衍生物均是潜在病毒抑制剂。 |
| Introduction | Remdesivir, or GS-5734, is an adenosine triphosphate analog first described in the literature in 2016 as a potential treatment for Ebola.1 In 2017, its activity against the coronavirus family of viruses was also demonstrated.2 Remdesivir is also being researched as a potential treatment to SARS-CoV2, the coronavirus responsible for COVID-19.4 |
| Application1 | Remdesivir是潜在冠状病毒抑制剂Experimental Unapproved Treatments for COVID-19 |
| Application2 | 瑞德西韦是一种核苷类似物,用于抑制RNA聚合酶的作用. |
| Application3 | 瑞德西韦是一种病毒抑制剂,在HAE 细胞中对 MERS-CoV和SARS-CoV的EC50 值均为74 nM,在延迟脑肿瘤细胞中对鼠肝炎病毒的EC50实验值为30nM。A nucleoside analog used to treat RNA virus infections. |
瑞德西韦(Remdesivir)是一种模拟核苷酸抑制剂,在体外对多种病毒具有有效的抗病毒活性,包括埃博拉病毒,马尔堡病毒,HCoV-NL63,HCoV-OC43,HCoV-229E,小鼠肝炎病毒(MHV),SARS-CoV,MERS-CoV 以及相关的蝙蝠冠状病毒,HKU5和PDCoV(猪三角洲冠状病毒)。 Remdesivir还据报道对2019年新型冠状病毒(称为2019-nCoV,nCoV2019或COVID-19)具有潜在的抗病毒活性。
Remdesivir或GS-5734是一种三磷酸腺苷类似物,于2016年首次在文献中描述为可能的埃博拉病毒治疗方法.2017年,它还被证明了对冠状病毒家族类病毒的活性有抑制作用。 SARS-CoV-2的潜在治疗方法,冠状病毒负责COVID-19。
Remdesivir, or GS-5734, is an adenosine triphosphate analog first described in the literature in 2016 as a potential treatment for Ebola.In 2017, its activity against the coronavirus family of viruses was also demonstrated.Remdesivir is also being researched as a potential treatment to SARS-CoV-2, the coronavirus responsible for COVID-19.
Remdesivir是一种核苷类似物,有望抑制RNA聚合酶的作用。通过掺入RNA不能添加额外的核苷酸,从而终止RNA转录。已证明RNA聚合酶突变的病毒对remdesivir产生部分抗性传染性较小。
Remdesivir is a nucleoside analog that is expected to inhibit the action of RNA polymerase.By incorporating into RNA, additional nucleotides cannot be added, terminating RNA transcription.Viruses with mutations in RNA polymerase to develop partial resistance to remdesivir have been shown to be less infective.
瑞德西韦研究进展:
1.准备在埃博拉疫情中使用的实验药物
检查海顿E.
国际卫生组织正在与刚果民主共和国讨论如何以及是否采用除疫苗之外的其他疗法。 国际卫生组织正在与刚果民主共和国讨论如何以及是否采用除疫苗之外的其他疗法。 刚果卫生部官员携带了第一批实验性埃博拉疫苗。
2.发现用于治疗埃博拉病毒的药物
Bixler SL,Duplantier AJ,Bavari S.
审查目的:
埃博拉病毒(Filoviridae家族)的成员,是人类严重病毒性出血热的病原体。在过去的40年中,该病毒与非洲几次高死亡率暴发有关,最近在西非暴发导致11,000多人死亡。这篇综述总结了埃博拉病毒病治疗药物的药物发现和开发过程的现状,重点是所使用的策略以及该过程各个阶段面临的挑战。最近发现:尽管有大量的体外功效数据,动物模型中的临床前数据以及人类临床数据,但尚未批准任何疗法可用于治疗埃博拉病毒病。但是,一些有希望的候选人,例如ZMapp和GS-5734,已经进入正在进行的临床试验。
瑞德西韦(GS-5734)在体外有效抑制MERS冠状病毒(MERS-CoV)复制,并在小鼠模型中显示出对严重急性呼吸系统综合症(SARS)-CoV的功效。在这里,我们 在MERS-CoV感染的非人类灵长类动物模型恒河猴中测试了预防和治疗性瑞姆昔韦治疗的功效。植入前24小时开始的预防性remdesivir治疗完全预防了MERS-CoV引起的临床 疾病后,严重抑制了呼吸系统中MERS-CoV的复制,并防止了肺部病变的形成。注入后12小时开始的雷姆昔韦韦治疗还提供了明显的临床症状,包括临床体征减少,肺部 此处提供的数据支持在MERS临床试验中对雷姆昔韦韦的治疗效果进行测试。还可以考虑将其用于更广泛的冠状病毒,包括 当前新兴的新型冠状病毒2019-nCoV。
BIOFOUNT公司目前衍生的瑞德西韦产品有:瑞德西韦一磷酸、瑞德西韦二磷酸、瑞德西韦三磷酸、瑞德西韦异构体等形式产品。
| Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S: Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016 Mar 17;531(7594):381-5. doi: 10.1038/nature17180. Epub 2016 Mar 2. [PubMed:26934220] |
| Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS: Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun 28;9(396). pii: 9/396/eaal3653. doi: 10.1126/scitranslmed.aal3653. [PubMed:28659436] |
| Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR: Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018 Mar 6;9(2). pii: mBio.00221-18. doi: 10.1128/mBio.00221-18. [PubMed:29511076] |
| de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H: Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020 Feb 13. pii: 1922083117. doi: 10.1073/pnas.1922083117. [PubMed:32054787] |
| Vander T, Medvedovsky M, Herishanu Y: Encephalopathy induced by oral acyclovir in a patient with normal renal function. J Infect. 2003 May;46(4):286. doi: 10.1053/jinf.2002.1119. [PubMed:12799156] |
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S: Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016 Mar 17;531(7594):381-5. doi: 10.1038/nature17180. Epub 2016 Mar
-
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS: Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun 28;9(396). pii: 9/396/eaal3653. doi: 10.1126/scitranslmed.aal3653. -
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR: Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018 Mar 6;9(2). pii: mBio.00221-18. doi: 10.1128/mBio.00221-18. -
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H: Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020 Feb 13. pii: 1922083117. doi: 10.1073/pnas.1922083117. -
Vander T, Medvedovsky M, Herishanu Y: Encephalopathy induced by oral acyclovir in a patient with normal renal function. J Infect. 2003 May;46(4):286. doi: 10.1053/jinf.2002.1119. -
Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, Hsueh PR: Arguments in favor of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020 Mar 5:105933. doi: 10.1016/j.ijantimicag.2020.105933. -
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T: Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020 Apr 10. doi: 10.1056/NEJMoa2007016. -
Ledford H: Hopes rise on coronavirus drug remdesivir. Nature. 2020 Apr 29. pii: 10.1038/d41586-020-01295-8. doi: 10.1038/d41586-020-01295-8.
※ Experimental drugs poised for use in Ebola outbreak
Check Hayden E.
International health organizations are in discussions with the Democratic Republic of Congo about how and whether to deploy treatments in addition to a vaccine. International health organizations are in discussions with the Democratic Republic of Congo about how and whether to deploy treatments in addition to a vaccine. Congolese Health Ministry officials carry the first batch of experimental Ebola vaccines.
※ Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase
Jordan PC, Liu C, Raynaud P, Lo MK, Spiropoulou CF, Symons JA, Beigelman L, Deval J.
Paramyxoviruses represent a family of RNA viruses causing significant human diseases. These include measles virus, the most infectious virus ever reported, in addition to parainfluenza virus, and other emerging viruses. Paramyxoviruses likely share common replication machinery but their mechanisms of RNA biosynthesis activities and details of their complex polymerase structures are unknown. Mechanistic and functional details of a paramyxovirus polymerase would have sweeping implications for understanding RNA virus replication and for the development of new antiviral medicines. To study paramyxovirus polymerase structure and function, we expressed an active recombinant Nipah virus (NiV) polymerase complex assembled from the multifunctional NiV L protein bound to its phosphoprotein cofactor. NiV is an emerging highly pathogenic virus that causes severe encephalitis and has been declared a global public health concern due to its high mortality rate. Using negative-stain electron microscopy, we demonstrated NiV polymerase forms ring-like particles resembling related RNA polymerases. We identified conserved sequence elements driving recognition of the 3'-terminal genomic promoter by NiV polymerase, and leading to initiation of RNA synthesis, primer extension, and transition to elongation mode. Polyadenylation resulting from NiV polymerase stuttering provides a mechanistic basis for transcription termination. It also suggests a divergent adaptation in promoter recognition between pneumo- and paramyxoviruses. The lack of available antiviral therapy for NiV prompted us to identify the triphosphate forms of R1479 and GS-5734, two clinically relevant nucleotide analogs, as substrates and inhibitors of NiV polymerase activity by delayed chain termination. Overall, these findings provide low-resolution structural details and the mechanism of an RNA polymerase from a previously uncharacterized virus family. This work illustrates important functional differences yet remarkable similarities between the polymerases of nonsegmented negative-strand RNA viruses.
※ Discovering Drugs for the Treatment of Ebola Virus
Bixler SL, Duplantier AJ, Bavari S.
PURPOSE OF REVIEW:
Ebola virus, a member of the Filoviridae family, is a causative agent of severe viral hemorrhagic fever in humans. Over the past 40 years, the virus has been linked to several high mortality outbreaks in Africa with the recent West African outbreak resulting in over 11,000 deaths. This review provides a summary of the status of the drug discovery and development process for therapeutics for Ebola virus disease, with a focus on the strategies being used and the challenges facing each stage of the process.
RECENT FINDINGS:
Despite the wealth of in vitro efficacy data, preclinical data in animal models, and human clinical data, no therapeutics have been approved for the treatment of Ebola virus disease. However, several promising candidates, such as ZMapp and GS-5734, have advanced into ongoing clinical trials.
SUMMARY:
The gravity of the 2014-2016 outbreak spurred a heightened effort to identify and develop new treatments for Ebola virus disease, including small molecules, immunotherapeutics, host factors, and clinical disease management options.
DISCLAIMER:
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endoresed by the U.S. Army.
※ Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS.
Emerging viral infections are difficult to control because heterogeneous members periodically cycle in and out of humans and zoonotic hosts, complicating the development of specific antiviral therapies and vaccines. Coronaviruses (CoVs) have a proclivity to spread rapidly into new host species causing severe disease. Severe acute respiratory syndrome CoV (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV) successively emerged, causing severe epidemic respiratory disease in immunologically naïve human populations throughout the globe. Broad-spectrum therapies capable of inhibiting CoV infections would address an immediate unmet medical need and could be invaluable in the treatment of emerging and endemic CoV infections. We show that a nucleotide prodrug, GS-5734, currently in clinical development for treatment of Ebola virus disease, can inhibit SARS-CoV and MERS-CoV replication in multiple in vitro systems, including primary human airway epithelial cell cultures with submicromolar IC50 values. GS-5734 was also effective against bat CoVs, prepandemic bat CoVs, and circulating contemporary human CoV in primary human lung cells, thus demonstrating broad-spectrum anti-CoV activity. In a mouse model of SARS-CoV pathogenesis, prophylactic and early therapeutic administration of GS-5734 significantly reduced lung viral load and improved clinical signs of disease as well as respiratory function. These data provide substantive evidence that GS-5734 may prove effective against endemic MERS-CoV in the Middle East, circulating human CoV, and, possibly most importantly, emerging CoV of the future.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻

怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33

提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07

各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28

荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11

如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



