-
法匹拉韦
NMR and HPLC COA下载 MSDS下载 - Names:
Favipiravir
- CAS号:
259793-96-9
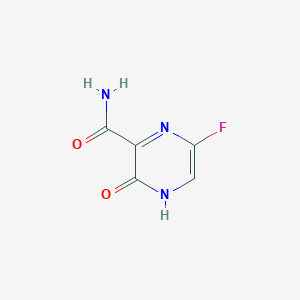
MDL Number: MFCD16879084 - MF(分子式): C5H4FN3O2 MW(分子量): 157.0287545
- EINECS: Reaxys Number:
- Pubchem ID: Brand:BIOFOUNT
法匹拉韦(Favipiravir,259793-96-9)是一种吡嗪甲酰胺衍生物,Favipiravir具有抗RNA病毒活性。Favipiravir 通过宿主酶转化为呋喃三磷酸核糖酯衍生物,可以选择性地抑制流感病毒RNA依赖的RNA聚合酶。
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| HCQ000001-1g | 1g | 98% | ¥ 1200.00 | ¥ 1200.00 | instock | ¥ 0.00 | ||
| HCQ000001-100mg | 100mg | 98% | ¥ 580.00 | ¥ 580.00 | instock | ¥ 0.00 |
| 中文别名 | 法维拉韦;法匹沙韦;法匹拉韦(CAS:259793-96-9,MSDS,应用,溶解度,),法匹拉韦杂质 |
| 英文别名 | Favipiravir,259793-96-9,Favipiravir (T-705),T 705,T-705,T705 |
| CAS号 | 259793-96-9 |
| Inchi | InChI=1S/C22H25BrN2O3S/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3/h6-11,26H,5,12-13H2,1-4H3 |
| InchiKey | KCFYEAOKVJSACF-UHFFFAOYSA-N |
| 分子式 Molecular Weight | C5H4FN3O2 |
| 分子量 Formula | 157.0287545 |
| 溶解度Solubility | 8.7 mg/mL,slightly soluble in water |
| 性状 | 固体粉末 |
| 储藏条件 Storage conditions | Store at room temperature |
法匹拉韦(CAS:259793-96-9,英文名:favipiravir)Protocol:
| 法匹拉韦细胞实验 | |
|---|---|
| Cell lines | MDCK cells, Vero cells, HEL cells, A549 cells, HeLa cells, and HEp-2 cells |
| Preparation method | The cytotoxicity of T-705 is evaluated by an assay with XTT. XTT is converted to aqueous formazan by an enzyme in MDCK cells, Vero cells, HEL cells, A549 cells, HeLa cells, and HEp-2 cells. The compounds are diluted to the appropriate concentrations (volume, 100 μl) with test medium (EMEM containing 10% FCS) in 96-well culture plates in which each well contains a concentration of 2 × 103 cells/100 μL. The test plates are incubated for 3 days at 37°C in 100% humidity and 5% CO2. After 3 days, 50 μl of the XTT reagent (1 mg/ml in FCS-free EMEM containing 5 mM phenazine methosulfate) is added, and the reaction product is assayed by measurement of the absorbance at 450 nm with a microplate reader. Cytotoxicity is expressed as the 50% cell-inhibitory concentration (CC50). |
| Concentrations | 1000 μg/mL |
| Incubation time | 3 d |
|
法匹拉韦动物实验
|
|
|---|---|
| Animal models | Mice infected with influenza virus A/PR/8/34 |
| Formulation | 0.5% methylcellulose |
| Dosages | 200 mg/kg/day |
| Administration | p.o. |
Conversion of different model animals based on BSA (Value based on data from FDA Draft Guidelines)
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of resveratrol used for a mouse (22.4 mg/kg) to a dose based on the BSA for a rat, multiply 22.4 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for resveratrol of 11.2 mg/kg.
Tags:法匹拉韦 试剂,法匹拉韦 抑制剂,法匹拉韦 衍生物,法匹拉韦 磷酸盐衍生物,法匹拉韦 杂质,法匹拉韦 中间体,法匹拉韦 公司,法匹拉韦 购买;法匹拉韦 供应商,
| 产品说明 | 法匹拉韦(259793-96-9,favipiravir)5-羟基-法匹拉韦,法匹拉韦一磷酸,法匹拉韦二磷酸,法匹拉韦三磷酸均为有效的病毒抑制剂 |
| Introduction | Favipiravir(259793-96-9)Favipiraviris triphosphate are initially approved for therapeutic use in resistant cases of influenza.7,9 The antiviral targets RNA-dependent RNA polymerase (RdRp) enzymes. |
| Application1 | 潜在冠状病毒抑制剂Experimental Unapproved Treatments for COVID-19,may be an alternative option for influenza strains that are resistant to neuramidase inhibitors.9,19 |
| Application2 | 法匹拉韦在人体内通过与糖作用生成“SCHEMBL7215591”,之后在结合磷酸盐生成法匹拉韦三磷酸形式产物,最终对病毒产生作用。 |
| Application3 | Favipiravir has been investigated for the treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19.10,14,15 |
法匹拉韦(CAS:259793-96-9,英文名:favipiravir)不仅可以抑制甲型和乙型流感病毒的复制,而且该药在禽流感的治疗中也有不错的疗效,并且可能是对神经酰胺酶抑制剂有抗药性的流感菌株的替代选择。可以作为治疗威胁生命的病原体,例如埃博拉病毒,拉沙病毒和现在的COVID-19
Favipiravir作为前药起作用,并在细胞内进行核糖基化和磷酸化,成为活性的Favipiravir-RTP。Favipiravir-RTP结合并抑制RNA依赖性RNA聚合酶(RdRp),最终可以阻止病毒转录和复制。
与现有的流感抗病毒药相比,favipiravir的作用机制是新颖的,主要能阻止病毒从细胞中进入和退出。活性的favipiravir-RTP选择性抑制RNA聚合酶并阻止病毒基因组的复制。目前有几种假设favipiravir-RTP如何与RNA依赖性RNA聚合酶(RdRp)相互作用。一些研究表明,将favipiravir-RTP掺入新生的RNA链中时,它会阻止RNA链延长和病毒增殖。7研究还发现, 嘌呤类似物可以降低favipiravir的抗病毒活性,表明favipiravir-RTP和嘌呤核苷之间存在RdRp结合竞争7。
尽管favipiravir最初是开发用于治疗流感的,但预计RdRp催化域(favipiravir的主要靶标)与其他RNA病毒相似。这种保守的RdRp催化域有助于favipiravir的广谱覆盖。
transcription of virus RNA segments. The transcription of viral mRNAs occurs by a unique mechanism called cap-snatching. 5' methylated caps of cellular mRNAs are cleaved after 10-13 nucleotides by PA. In turn, these short capped RNAs are used as primers by PB1 for transcription of viral mRNAs. During virus replication, PB1 initiates RNA synthesis and copy vRNA into complementary RNA (cRNA) which in turn serves as a template for the production of more vRNAs.Favipiravir shows anti-influenza virus activities with IC50 ranged from 0.013 to 0.48 μg/ml for the influenza A viruses, from 0.039 to 0.089 μg/ml for the influenza B viruses, and from 0.030 to 0.057 μg/ml for the influenza C viruses. In mammalian cell lines (MDCK cells, Vero cells, HEL cells, A549 cells, HeLa cells, and HEp-2 cells), Favipiravir shows no cytotoxicity at concentrations up to 1,000 μg/ml. In MDCK cells inoculated with seasonal influenza A (H1N1) viruses, Favipiravir induces lethal mutagenesis.
| Beigel J, Bray M: Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. doi: 10.1016/j.antiviral.2008.01.003. Epub 2008 Feb 4. [PubMed:18328578] |
| Hsieh HP, Hsu JT: Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des. 2007;13(34):3531-42. [PubMed:18220789] |
| Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW: In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007 Sep;51(9):3168-76. Epub 2007 Jul 2. [PubMed:17606691] |
| Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y: Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007 Mar;51(3):845-51. Epub 2006 Dec 28. [PubMed:17194832] |
| Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K: Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005 Mar;49(3):981-6. [PubMed:15728892] |
Beigel J, Bray M: Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. doi: 10.1016/j.antiviral.2008.01.003. Epub 2008 Feb 4.
Hsieh HP, Hsu JT: Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des. 2007;13(34):3531-42.
Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW: In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007 Sep;51(9):3168-76. Epub 2007 Jul 2.
Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y: Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007 Mar;51(3):845-51. Epub 2006 Dec 28.
Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K: Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005 Mar;49(3):981-6.
Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K: In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002 Apr;46(4):977-81.
Furuta Y, Komeno T, Nakamura T: Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449-463. doi: 10.2183/pjab.93.027.
Venkataraman S, Prasad BVLS, Selvarajan R: RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses. 2018 Feb 10;10(2). pii: v10020076. doi: 10.3390/v10020076.
Hayden FG, Shindo N: Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019 Apr;32(2):176-186. doi: 10.1097/QCO.0000000000000532.
Madelain V, Nguyen TH, Olivo A, de Lamballerie X, Guedj J, Taburet AM, Mentre F: Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet. 2016 Aug;55(8):907-23. doi: 10.1007/s40262-015-0364-1.
Nguyen TH, Guedj J, Anglaret X, Laouenan C, Madelain V, Taburet AM, Baize S, Sissoko D, Pastorino B, Rodallec A, Piorkowski G, Carazo S, Conde MN, Gala JL, Bore JA, Carbonnelle C, Jacquot F, Raoul H, Malvy D, de Lamballerie X, Mentre F: Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017 Feb 23;11(2):e0005389. doi: 10.1371/journal.pntd.0005389. eCollection 2017 Feb.
de Farias ST, Dos Santos Junior AP, Rego TG, Jose MV: Origin and Evolution of RNA-Dependent RNA Polymerase. Front Genet. 2017 Sep 20;8:125. doi: 10.3389/fgene.2017.00125. eCollection 2017.
Shu B, Gong P: Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):E4005-14. doi: 10.1073/pnas.1602591113. Epub 2016 Jun 23.
Nagata T, Lefor AK, Hasegawa M, Ishii M: Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015 Feb;9(1):79-81. doi: 10.1017/dmp.2014.151. Epub 2014 Dec 29.
Rosenke K, Feldmann H, Westover JB, Hanley PW, Martellaro C, Feldmann F, Saturday G, Lovaglio J, Scott DP, Furuta Y, Komeno T, Gowen BB, Safronetz D: Use of Favipiravir to Treat Lassa Virus Infection in Macaques. Emerg Infect Dis. 2018 Sep;24(9):1696-1699. doi: 10.3201/eid2409.180233. Epub 2018 Sep 17.
Delang L, Abdelnabi R, Neyts J: Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018 May;153:85-94. doi: 10.1016/j.antiviral.2018.03.003. Epub 2018 Mar 7.
Nature Biotechnology: Coronavirus puts drug repurposing on the fast track
Pharmaceuticals and Medical Devices Agency: Avigan (favipiravir) Review Report
World Health Organization: Influenza (Avian and other zoonotic)
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
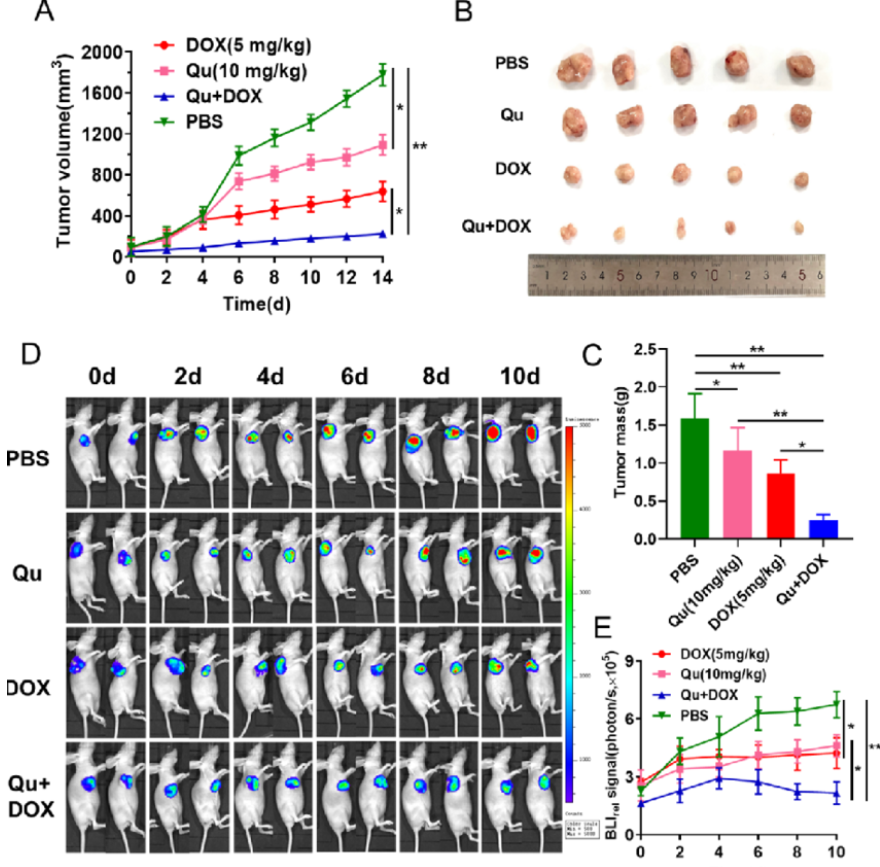
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



